CS 2019.02.01.8
Files > Conference Series > 2019 > Humboldt Kolleg 2019
Bionatura Conference Series Vol 2. No 1. 2019
“Breaking Paradigms: Towards a Multi-, Inter- and Transdisciplinary Science” In commemoration of the 250th Anniversary of Alexander von Humboldt
Relationship between Charge Transfer Diffusion Coefficients and Doping Level for electro generated thin PEDOT films on ITO
Alex Palma-Candoab*, Bernardo A. Frontana-Uribeb,c*, Victor Varela-Guerrerob
Available in : http://dx.doi.org/10.21931/RB/CS/2019.02.01.8
ABSTRACT
Thin films of poly-3,4-ethylene dioxythiophene (PEDOT) were electrodeposited on transparent electrodes of indium tin oxide (ITO) using potentiostatic regime. These films had thicknesses ranging from 15 nm to 60 nm and were studied using UV-vis absorption and chronoamperometric techniques in monomer-free tetrabutylammonium perchlorate/acetonitrile solutions. The charge transfer diffusion coefficient (D) of the films were calculated using Cottrell model for a wide range of potential steps from −1.60 to 1.60 V vs. Ag°/AgNO3. For p-doped films, the highest diffusion coefficients were obtained when a potential of 0.70 V was applied. Moreover, a direct relationship between film thickness and their diffusion coefficients was found for thin PEDOT films showing D values up to 1.4 × 10−9 cm2 s−1 for 60 nm thickness films. These values are remarkably higher than the D of 1.8 × 10−11 cm2 s−1 obtained for spin-coated PEDOT: PSS films of similar thickness.
Keywords: Electropolymerization, PEDOT, thin films, diffusion coefficient.
INTRODUCTION
One of the most popular polythiophenes in the field of organic electronic devices is the poly-3,4,-ethylene dioxythiophene (PEDOT) in the form of a polymer mixture with polystyrene sulfonate (PSS) (see Scheme 1a), which for the last two decades has been intensively studied because of its high conductivity and excellent stability.1-5 Thin films of p-doped PEDOT show good transparency in the UV-vis region. For these reasons, thin PEDOT films have found different applications in devices such as sensors, antistatic coatings, organic light-emitting diodes (OLEDs), electrochromic windows as well as in organic photovoltaic devices mainly used as hole extraction layers. 6, 7

Scheme 1. Chemical structures for a) PEDOT: PSS and b) PEDOT: ClO4−
Furthermore, thin PEDOT films can be obtained from EDOT solutions on suitable electrodes (e.g., indium tin oxide) by electrochemical polymerization.8-10 Electropolymerization allows not only a controlled polymerization and deposition of the film in one-step but also a precise control of the thickness and conductive characteristics of the film by tuning some electrochemical parameters (e.g., the potential of polymerization, doping level).11-15 Moreover, electrogenerated thin PEDOT films, containing small counteranions, like perchlorate (PEDOT: ClO4−, Scheme 1b), have shown higher conductivities than thin PEDOT: PSS films deposited from aqueous solutions of PEDOT: PSS by spin-coating.16
Charge transport of conducting polymers is related to the microstructure of thin films.17 Since the length of the main chain of electrogenerated polymers is short (mostly oligomers), charge carriers (e.g., electronic or ionic particles) do not move on a continuous intramolecular pathway; instead, intermolecular transport of the charges is the dominant process.18 During the p-doping process of a conducting polymer, electrons are taken out from the thin film (oxidation process) and anions are simultaneously inserted in the thin film to preserve the electroneutrality of the material. For this reason, each positive electronic charge (e.g., hole) in the thin film, is counterbalanced by an anion which boosts the polymer conductivity. Therefore, a higher p-doping level implies an increase in the concentration of holes and anions in the conducting polymer thin film. The conductivity of the material can be attributed to the mobility of these species, which are charge carriers, along the conjugated backbone.
Charge transport properties, the in homogeneous process of diffusion/migration through conducting polymer films, can be quantified by the charge transfer diffusion coefficient (D) using the Cottrell model (vide infra). Nevertheless, it is not possible to know with this model the contribution made by diffusion and migration separately. Those diffusion coefficients could provide a different approach and understanding of electrical properties of conducting polymers. Diffusion coefficients are directly related to the charge carriers’ mobility and conductivity for different materials (e.g. crystalline semiconductors) via the Einstein equation.19 However, it has been reported that this equation only can be regarded as an indicator of the degree of dispersion of conjugated polymers because of their complex morphology. 20 Additional equations have been considered in which case conductivity is proportional to D1/2.21
Charge transfer diffusion coefficients can be determined by using chronoamperometric techniques to quantitatively describe charge transport in conducting films. The resultant current (i) can be measured as a function of time (t) by means of the classic Cottrell equation for chronoamperometry, 21

where Q is the total consumed charge during the film oxidation and L is the film thickness. Diffusion coefficients calculated using the Cottrell equation were reported by Ito and co-workers for PEDOT: PSS films on ITO prepared by a layer-by-layer deposition technique. 22 These films showed D values up to 5 × 10−10 cm2 s−1. Here, we aim to determine charge transfer diffusion coefficients of electrochemically generated thin PEDOT films on ITO, following the reported methodology described by Ito and co-workers, 22 throughout a large range of potential steps resulting in polymer films at different doping levels.
In this study, UV-vis absorption behavior of electrogenerated thin PEDOT films is related to their charge transfer diffusion coefficients determined by chronoamperometric techniques, within a wide range of potential steps from −1.60 V to 1.60 V vs. Ag°/AgNO3. Highly p-doped PEDOT films are obtained when potentials of −0.10 V or higher are applied, showing maximum values of D at a potential of 0.70 V. However, when doping potentials higher than 1.00 V are applied the diffusion coefficients drastically decrease, this behavior is probably due to overoxidation of the polymer. A direct relationship between the thickness of thin PEDOT films and the diffusion coefficients is shown. Finally, diffusion coefficients of electrogenerated PEDOT films are compared to spin-coated PEDOT: PSS, which are typically used in organic electronic devices.
Experimental Section
ITO electrodes were purchased from KINTEC (10-ohm m−2, Polished grade, 25 × 10 × 1.1 mm); 3,4-ethylenedioxythiophene (EDOT), anhydrous acetonitrile (MeCN, 99.8% purity), PEDOT: PSS (conductive grade, water dispersion 1.3 wt%) and tetrabutylammonium perchlorate (TBAP, ≥ 99% purity) from ALDRICH. All the chemicals were used as received. Thin PEDOT films were electrodeposited on ITO electrodes as previously reported.23,24 The absorption spectra of the films were measured on a UV-vis spectrophotometer THERMO SCIENTIFIC GENESYS 10S UV-vis. Charge transfer diffusion coefficients of thin PEDOT films were calculated from experiments in monomer-free solutions under nitrogen atmosphere at 25 °C. A three-electrode cell was used with ITO/PEDOT electrode (area of 1.0 cm2) as working electrode (WE), platinum mesh as a counter electrode (CE) (area of 2.9 cm2), and Ag°/AgNO3 as a reference electrode (RE). The WE and CE were placed in parallel separated by a distance of 1.1 cm. Chronoamperometric pulses were applied at various pre-potentials for a time of 30 s, and at several final potentials for a time of 1 s, within a range of −1.60 V to 1.60 V. Current transients for thin PEDOT films were plotted for different potential steps. These plots were used to determine the charge transfer diffusion coefficients using the Cottrell model (Equation 1).
RESULTS AND DISCUSSION
Thin PEDOT films with a thickness of ca. 60 nm were deposited on ITO as reported previously. 23,24 Absorption spectra of thin PEDOT films during electrochemical p-doping and un doping, in 0.1 M TBAP/MeCN solution, is shown in Figure 1. The spectrum for the "as deposited" thin PEDOT film showed an absorbance maximum in the UV region of 360 nm, and a shoulder in the visible region around 700 nm. A series of UV-vis spectra of thin PEDOT films, measured after applying various potential pulses from −1.40 V to 1.00 V, shows a progressive decreasing in absorbance of the peak located at 600 nm. This peak is related to the π-π* transition for neutral PEDOT. Furthermore, a continuous increasing absorbance of the 950 nm signal due to the formation of radical cations (polarons) and dications (bipolarons). 25 Hence, the structure of thin PEDOT films changed from a neutral state to a highly conducting p-doped state. A comparison of “as deposited” spectrum and highly oxidized PEDOT spectra demonstrate that the initial PEDOT film was already highly p-doped. When oxidation potentials higher than 1.0 V were applied, a general decrease in absorbance was shown for the UV-vis spectra but any significant change was observed in the form of the spectra. This behavior could be assigned to the overoxidation when the potential is over 1.0 V with the resulting degradation of the thin PEDOT films.

Figure 1. Absorption spectra of PEDOT deposits with a thickness of 60 nm on ITO after various potential pulses.
For a better appreciation and a quantitative discussion of charge (e.g., holes and counteranions) transport properties of thin PEDOT films on ITO, charge transfer diffusion coefficients (D) were calculated using the Cottrell model. Figure 2 shows current-time curves for PEDOT deposit with a thickness of ca. 60 nm on ITO as well as bare ITO electrode (inset Figure 2). The current transients were obtained after applying a potential of 0.80 V during 1 s with a pre-potential of −1.00 V for 30 s. A much smaller current was observed for a bare ITO electrode (related to the double layer charging) compared to the current shown by the PEDOT film, which is mainly assigned to the doping process of the conducting polymer. A plot of current vs. inverse square root of time shows a linear relation for short periods of time. The charge transfer diffusion coefficient is calculated using the slope of the linear plot.

Figure 2. Current transient for bare ITO and thin PEDOT films with a thickness of 60 nm on ITO when a doping potential of 0.80 V is applied for 1 s after a pre-potential of −1.00 V applied for 30 s. The inset shows the curve for a bare ITO electrode.
Charge transfer diffusion coefficients of thin PEDOT films were obtained applying a highly p-doping potential of 0.80 V during 1s, after a series of pre-potentials from −1.60 V to 0.70 V were applied during 30 s. Thereby, PEDOT films deposited on ITO, submitted to different potential pulse, were studied ranging from undoped films (e.g., for pre-potentials of −1.60 V) to highly p-doped deposits (e.g., for pre-potentials of 0.70 V). In other words, both large and small structural changes were studied for thin PEDOT films. The related chronoamperograms are depicted in Figure 3a. Similar trends are observed regardless of the potential step applied. The current might mainly originate from the oxidation process of thin PEDOT films, that is, the structural changes on these thin films have none or imperceptible contribution to the current. Figure 3b shows the charge transfer diffusion coefficients calculated using the Cottrell model (Equation 1).

Figure 3. (a) Chronoamperograms for thin PEDOT films with thickness of 60 nm, and (b) charge transfer diffusion coefficients (D) as a function of pre-potentials (Ei) applied during 30 s. These prepotentials were followed by the application of a potential of 0.80 V during 1 s.
Throughout the application of different potential amplitudes, the charge transfer diffusion coefficients remained almost invariable. An average D of 1.4 × 10−9 cm2 s−1 with a standard deviation of 0.1 × 10−9 cm2 s−1 were determined for thin PEDOT films. The calculated diffusion coefficient was higher than those reported for PEDOT: PSS films which were deposited by the layer-by-layer technique of 5 × 10−10 cm2 s−1 22 and spin-coating technique of 6 × 10−10 cm2 s−1. 26
For the next section of the study, diffusion coefficients were calculated applying a pre-potential of −0.90 V during 30 s, followed by different potentials ranging from −0.80 V to 1.60 V for a time of 1 s. The current transients for potentials ≤ 1.00 V quickly decrease to values close to zero (Figure 4a). However, thin PEDOT films overoxidation may be observed on the chronoamperograms for potentials ≥ 1.20 V, where the current transients do not tend to zero. This amount of remaining current might be assigned to PEDOT overoxidation and charges trapped into the polymer film. The former is confirmed by the UV-vis absorption spectra of thin PEDOT films doped at several potentials (see Figure 1).

Figure 4. (a) Chronoamperograms for thin PEDOT films with a thickness of 60 nm and (b) charge transfer diffusion coefficients (D) as a function of various potentials (Ef) applied during 1 s. These potentials followed the application of a pre-potential of −0.90 V during 30 s.
Figure 4b shows charge transfer diffusion coefficients calculated by Cottrell model (Equation 1). Two areas are clearly observed in the plot, (i) D for potentials between −0.10 V and 1.60 V increased following a parabolic trend whereby maximum diffusion coefficient values were obtained for a potential range of ca. 0.70 V. Similar trends have been reported for in situ conductivity measurements,27 where the maximum conductivity is not necessarily found at the maximum applied potential. When potentials higher than −0.10 V was applied, UV-vis absorption spectra of thin PEDOT films showed the peak assigned to the π-π* transitions to be overlaid by polaronic and bipolaronic waves (see Figure 1). This confirms that highly doped thin PEDOT films are obtained at this potential range. However, D values quickly decreased when potentials higher than 1.20 V were applied. The decrease in the charge transfer diffusion coefficients is attributed to overoxidation process of thin PEDOT films, which is confirmed by UV-vis absorption and chronoamperometric studies. These potential values probably change the chemical structure and properties of the PEDOT deposits, e.g., decrease conductivity. (ii) For potentials lower than −0.10 V, the values of D did not follow the trend showed for higher p-doping potentials. This phenomenon, expected for undoped or barely doped polymer structures, is observed because Cottrell model fails when any diffusion of charges flows into the polymer.
Figure 5 depicts cyclic voltammograms, for PEDOT deposits of 60 nm thickness, obtained by applying an initial potential of −1.00 V and switching potentials of 1.00 V and 1.35 V with a scan rate of 0.10 Vs−1 in monomer-free solution, and charge transfer diffusion coefficients taken from Figure 4b. Similar as reported in the literature for conductivity measurements,28 the polymer oxidation peak centered at ca. 0.10 V seems to be the breakpoint between an undoped or barely doped, and a highly doped conducting structure. Moreover, the maximum value of diffusion coefficients is found to be on the voltammogram plateau for a potential of 0.70 V. This behavior was also previously reported for PEDOT deposits using in situ conductivity measurements.29, 30 Structural changes are clearly identified by the polymer oxidation and reduction peak shifts, and the change in the shape of the cyclic voltammograms obtained at switching potential of 1.00 V and 1.35 V (Figure 5).
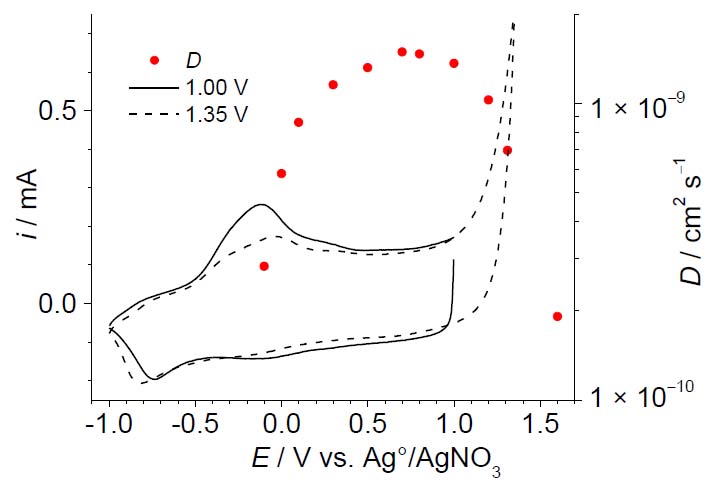
Figure 5. Cyclic voltammograms and charge transfer diffusion coefficients (D) of thin PEDOT films in monomer-free solution. The CVs were obtained applying an initial potential of −1.00 V and switching potentials of 1.00 V (solid line) and 1.35 V (dashed line) with a scan rate of 0.10 Vs−1.
Charge transfer diffusion coefficients were determined for electrogenerated thin PEDOT films of three different thicknesses of 15, 30 and 60 nm doped with three different counter anions of perchlorate, hexafluorophosphate, and tetrafluoroborate, as well as PEDOT: PSS spin-coated on ITO with a thickness of ca. 60 nm (see Table 1). For this purpose, D was calculated by applying a potential of 0.80 V during 1 s with a pre-potential of 0.5 V for a time of 30 s too thin PEDOT films in monomer-free solution.

Table 1. Charge transfer diffusion coefficients (D) of electrogenerated PEDOT films with thicknesses of 15, 30 and 60 nm doped with perchlorate, hexafluorophosphate and tetrafluoroborate anions, and PEDOT: PSS spin-coated on ITO with a thickness of ca. 60 nm. D values were determined to apply a pre-potential of 0.50 V for a time of 30 s followed by a potential of 0.80 V during 1s.
A direct relationship was found between the film thickness of electrogenerated PEDOT deposits and their corresponding diffusion coefficients. An increased film thickness results in increased charge transfer diffusion coefficients. Similar behavior for thin PEDOT films was reported by Park and co-workers, 31 who found a similar relationship between thickness and conductivity. Lower diffusion coefficient values for thinner films can be assigned to early growing stages of polymer structures due to the formation of mainly short chains of primary oligomers. As the film grows, a longer polymer conjugation is obtained through longer polymer chains until the polymer thickness reaches a certain level, and at this point, the conductivity decreases while the thickness increases. Furthermore, any significative influence in the diffusion coefficients was observed by doping electrogenerated PEDOT films with different counteranions. Finally, charge transfer diffusion coefficients for electrogenerated thin PEDOT films were found to be higher than spin-coated PEDOT: PSS films of similar thicknesses. A similar behavior was reported based on conductivity measurements,16 ascribing this phenomenon to the presence of PSS non-electronic conductor macromolecules in the PEDOT bulk.32 PSS macromolecules impact on the diffusion coefficient, because the charge transfer in a conducting polymer occurs mainly as an interchain process,18 thus the PSS non-electronic conductor macromolecules in the PEDOT bulk leads to a decrease in conductivity if compared to thin PEDOT films that contain only small counteranions, as perchlorate. These types of small counterions do not interfere with charge transport. Larger D values lead to higher conductivities which is very important for technological applications.
CONCLUSIONS
Charge transfer diffusion coefficients of electrodeposited PEDOT films on ITO with a thickness of 60 nm were determined for a large range of potential steps in a monomer-free solution. The diffusion coefficients were obtained by chronoamperometric techniques using Cottrell model. By means of UV-vis absorption spectra and diffusion coefficients, thin PEDOT films are found in undoped or barely doped state (lower than −0.10 V), highly doped state (−0.10 V to 1.0 V) and overoxidized state (higher than 1.0 V). The highest diffusion coefficients were obtained for potentials ca. 0.70 V. Moreover, diffusion coefficients of thin PEDOT films show a direct relationship with their film thickness (15, 30 and 60 nm). Finally, two orders of magnitude higher charge transfer diffusion coefficients are shown for electrogenerated PEDOT films if compared to spin-coated PEDOT: PSS of similar thicknesses (ca. 60 nm). The large difference in D values was attributed to the introduction of PSS non-electronic conductor macromolecules on the conducting polymer bulk, where the phase separation can generate lower conductivity values.
Acknowledgments
We gratefully acknowledge financial support by the CONACyT (Mexico) through project No. 179356. A. U. Palma-Cando thanks the Secretaría de Relaciones Exteriores (SRE) of Mexico for the scholarship No. 7446.
REFERENCES
1. Groenendaal L, Jonas F, Freitag D, Pielartzik H, Reynolds JR (2000) Poly(3,4-ethylenedioxythio-phone) and Its Derivatives: Past, Present, and Future. Adv Mater 12:481-494
2. Liu H, Zhou W, Ma X, Chen S, Ming S, Lin K, Lu B, Xu J (2016) Capacitive performance of electrodeposited PEDOS and a comparative study with PEDOT. Electrochim Acta 220:340-346
3. Ouyang L, Wei B, Kuo Cc, Pathak S, Farrell B, Martin DC (2017) Enhanced PEDOT adhesion on solid substrates with electrografted P(EDOT-NH2). Science Advances DOI: 10.1126/sciadv.1600448
4. Liu Y, Zhao Y, Xu S, Cao S (2015) Enhanced electroluminescent efficiency with ionic liquid doped into PEDOT:PSS hole-injecting layer. Polymer 77:42-47
5. Le Ouay B, Boudot M, Kitao T, Yanagida T, Kitagawa S, Uemura T (2016) Nanostructuration of PEDOT in Porous Coordination Polymers for Tunable Porosity and Conductivity. J Am Chem Soc 138:10088-10091
6. Po R, Carbonera C, Bernardi A, Camaioni N (2011) The role of buffer layers in polymer solar cells. Energy Environ Sci 4:285-310
7. Wu Y, Wang J, Qiu X, Yang R, Lou H, Bao X, Li Y (2016) Highly Efficient Inverted Perovskite Solar Cells With Sulfonated Lignin Doped PEDOT as Hole Extract Layer. ACS Appl Mater Interfaces 8:12377-12383
8. Melato AI, Viana AS, Abrantes LM (2008) Different steps in the electrosynthesis of poly(3,4-ethylene dioxythiophene) on platinum. Electrochim Acta 54:590-597
9. Vasantha VS, Thangamuthu R, Chen SM (2008) Electrochemical Polymerization of 3,4-Ethylenedioxythiophene from Aqueous Solution Containing Hydroxypropyl-β-cyclodextrin and the Electrocatalytic Behavior of Modified Electrode Towards Oxidation of Sulfur Oxoanions and Nitrite. Electroanalysis 20:1754-1759
10. Nasybulin E, Wei S, Cox M, Kymissis I, Levon K (2011) Morphological and Spectroscopic Studies of Electrochemically Deposited Poly(3,4-ethylene dioxythiophene) (PEDOT) Hole Extraction Layer for Organic Photovoltaic Device (OPVd) Fabrication. J Phys Chem C 115:4307-4314
11. Ibanez JG, Rincón ME, Gutierrez-Granados S, Chahma Mh, Jaramillo-Quintero OA, Fontana-Uribe BA (2018) Conducting Polymers in the Fields of Energy, Environmental Remediation, and Chemical–Chiral Sensors. Chem Rev 118:4731-4816
12. Inzelt G (2017) Recent Advances in the Field of Conducting Polymers. J Solid State Electrochem 21:1965-1975
13. Palma-Cando A, Scherf U (2015) Electrogenerated Thin Films of Microporous Polymer Networks with Remarkably Increased Electrochemical Response to Nitroaromatic Analytes. ACS Appl Mater Interfaces 7:11127-11133
14. Palma-Cando A, Scherf U (2016) Electrochemically Generated Thin Films of Microporous Polymer Networks: Synthesis, Properties, and Applications. Macromol. Chem Phys 217:827-841
15. Palma-Cando A, Woitassek D, Brunklaus G, Scherf U (2017) Luminescent Tetraphenylethene-Cored, Carbazole- and Thiophene-Based Microporous Polymer Films for the Chemosensing of Nitroaromatic Analytes. Mater Chem Front 1:1118-1124
16. Lee HJ, Lee J, Park SM (2010) Electrochemistry of Conductive Polymers. 45. Nanoscale Conductivity of PEDOT and PEDOT:PSS Composite Films Studied by Current-Sensing AFM. J Phys Chem B 114:2660-2666
17. Roncali J (1997) Synthetic Principles for Bandgap Control in Linear pi-Conjugated Systems. Chem Rev 97:173-206
18. Rubinson JF, Kayinamura YP (2009) Charge transport in conducting polymers: insights from impedance spectroscopy. Chem Soc Rev 38:3339-3347
19. Kubo R, Toda M, Hashitsume N (1991) Statistical Physics II, 2nd edn., Springer-Verlag Berlin Heidelberg
20. Tseng HE, Jen TH, Peng KY, Chen SA (2004) Measurements of charge mobility and diffusion coefficient of conjugated electroluminescent polymers by time-of-flight method. Appl Phys Lett 84:1456-1458
21. Lyons M (1994) In: M. Lyons (ed) Electroactive Polymer Electrochemistry, Plenum Press, New York
22. Wakizaka D, Fushimi T, Ohkita H, Ito S (2004) Hole transport in conducting ultrathin films of PEDOT/PSS prepared by layer-by-layer deposition technique. Polymer 45:8561-8565
23. Palma-Cando A, Frontana-Uribe BA, Maldonado JL, Rivera-Hernández M (2014) Control of Thickness of PEDOT Electrodeposits on Glass/ITO Electrodes from Organic Solutions and its Use as Anode in Organic Solar Cells. Procedia Chem 12:92-99
24. Palma-Cando A, Rivera-Hernández M, Frontana-Uribe BA (2013) Electrodepósitos de Poli-3,4-etilendioxitiofeno sobre electrodos transparentes de Oxido de Indio y Estaño. Control del Espesor y Morfología. Química Central, 3:43-51
25. Chen X, Inganäs O (1996) Three-Step Redox in Polythiophenes: Evidence from Electrochemistry at an Ultramicroelectrode. J Phys Chem 100:15202-15206
26. Ghosh S, Inganäs O (1999) Self-assembly of a Conducting Polymer Nanostructure by Physical Crosslinking: Applications to Conducting Blends and Modified Electrodes. Synth Met 101:413-416
27. Heinze J, Frontana-Uribe BA, Ludwigs S (2010) Electrochemistry of Conducting Polymers-Persistent Models and New Concepts. Chem Rev 110:4724-4771
28. Łapkowski M, Proń A (2000) Electrochemical oxidation of poly(3,4-ethylenedioxythiophene) - "in situ" conductivity and spectroscopic investigations. Synth Met 110:79-83.
29. Aubert PH, Groenendaal L, Louwet F, Lutsen L, Vanderzande D, Zotti G (2002) In situ conductivity measurements on polyethylenedioxythiophene derivatives with different counterions. Synth Met 126:193-198
30. Zotti G, Zecchin S, Schiavon G, Groenendaal LB (2000) Conductive and Magnetic Properties of 3,4-Dimethoxy- and 3,4-Ethylenedioxy-Capped Polypyrrole and Polythiophene. Chem Mater 12:2996-3005
31. Han DH, Kim JW, Park SM (2006) Electrochemistry of Conductive Polymers 38. Electrodeposited Poly(3,4-ethylenedioxy-thiophene) Studied by Current Sensing Atomic Force Microscopy. J Phys Chem B 110:14874-14880
32. Zotti G, Zecchin S, Schiavon G, Louwet F, Groenendaal L, Crispin X, Osikowicz W, Salaneck W, Fahlman M (2003) Electrochemical and XPS Studies toward the Role of Monomeric and Polymeric Sulfonate Counterions in the Synthesis, Composition, and Properties of Poly(3,4-ethylenedioxythiophene). Macromolecules 36:3337-3344
Received: 3 March 2019
Accepted: 28 May 2019
Alex Palma-Candoab*, Bernardo A. Frontana-Uribeb,c*, Victor Varela-Guerrerob
a Escuela de Ciencias Químicas e Ingeniería, Universidad Yachay Tech, 100115 Urcuquí, Ecuador
b Centro Conjunto de Investigación en Química Sustentable UAEMex-UNAM, 50200 Toluca, México.
c Instituto de Química – UNAM, Circuito exterior S/N Ciudad Universitaria, 04510 Ciudad de México, México.
Corresponding authors: *[email protected], *[email protected]
