2023.08.04.89
Files > Volume 8 > Vol 8 no 4 2023
Haider A. Hassan1*, Saad Hasan Mohammed Ali2, Athraa Y. Al-hijazi3
1Biotechnology Department, University of Baghdad, Iraq.
2 College of Medicine, University of Baghdad, Clinical Communicable Disease Research Unit, Iraq
3Oral histology and biology, al-Mustaqbal University, Iraq Corresponding author: [email protected] Available from: http://dx.doi.org/10.21931/RB/2023.08.04.89
ABSTRACT
Heat shock protein70 (HSP70) is a crucial protein with vital biological tasks to continue cell life. The variation of HSP70 activation occurs due to stress, which includes temperature states, toxicity, poisoning with heavy metal, and tumor-related conditions. One of the master jobs of the HSP family is the suppression of the apoptosis signals, which are caspase-mediated. A high level of the expression of HSP70 is accountable for tumorigenesis and resistance against chemotherapeutic drugs. For this reason, the detection of HSP70 may help to diagnose cancerous diseases.
Conversely, targeting this chaperone might help treatment by maintaining late caspase‐dependent events. This study was conducted to detect the presence and the location of HSP70 in Iraqi thyroid cancer tissue specimens (25 samples) and (10 samples) of normal thyroid tissue. Using an immunohistochemical study (paraffin method), the protein was detected in 100% of follicular carcinoma or follicular adenoma in addition to 77.7 % of papillary thyroid carcinoma, while in normal thyroid tissue, the presence of protein was in 10% of cases. Regarding protein location in the cells, it appeared in the nuclei and the cytoplasm of follicular carcinoma cases compared to just in the cytoplasm of other cases.
Keywords: Thyroid, cancer, Heat shock protein70, immunohistochemical expression.
INTRODUCTION
Thyroid cancer (T.C.) is the most frequent type of endocrine malignancy (3.4 % of all cancers diagnosed annually). The incidence rates of both males and females are five times higher in high and very high Human Development Index (HID) countries than in low and medium (HID) countries1,2. Several types and subtypes with different cellular origins, characteristics and prognoses are present in thyroid cancerous lesions. Three chief histological types of T.C. were detected: differentiated (papillary and follicular) T.C., undifferentiated (poorly differentiated and anaplastic T.C.) and medullary TC3. Approximately 90 % of all T.C.s originate from thyroid follicular cells, which are well-differentiated tumors (DTCs), and the type considered the most common histological type of these cancers is papillary thyroid carcinoma (PTC), followed by follicular thyroid carcinoma4. In terms of T.C. treatment, there are three options, which include total thyroidectomy, which means getting rid of the entire thyroid gland, therapy with iodine radioactive isotopes, and therapies that target specific molecules that inhibit the tyrosine kinase pathway. Differentiated T.C. might respond to surgical resection, radioiodine ablation (RAI), and therapy via suppression of hormones that stimulate thyroid5. In contrast, patients with poorly differentiated tumors that are unresponsive to conventional therapies have a worse prognosis; this subgroup of cases is obstinate to therapy with chemicals and therapy with radiation and usually develops recurrent disease and metastatic invasion6. Chaperone machines mediate many intracellular processes. One of the most essential chaperone machines is the heat shock protein 70 (HSP70) family, which mediates crucial processes such as protein folding, modification, translocation, and cell survival. When stress occurs, the cell must deal with raised concentrations of unfolded or collapsed proteins when the HSP70 level increases to help in this function7. Commonly, HSP70 translocation occurs into nucleoli from the nucleus and cytoplasm using microtubules under kinase enzyme action. The fast translocation of Hsp70, particularly during heat shock, is thought to provide fast folding and refolding of denatured and aggregated proteins8 .Thus, HSP70 is overexpressed in various malignancies such as prostate, lung, pancreatic, urothelial, cervical, and breast cancers9. HSP70 prevents protein aggregation and assists the translocation of other proteins through the membrane, which has been explored10.
Furthermore, formulation of HSP70 in the cells can interfere with different biological processes such as inhibition of both apoptosis- intrinsic and extrinsic as cell death pathways11, senescence12, autophagy-13,14. Regarding the innate immune system, HSP70 may be a danger signal promoting receptor-mediated apoptosis. HSP70 is overexpressed in cells by stress, which can cause acquired or inherent drug resistance7. Indeed, such overexpression may be considered for tumorigenesis and opposition chemotherapeutic agents used in treatment. This process occurs via prohibiting late caspase‐dependent signal15. This work investigated the expression pattern of HSP70 in T.C. patients to clarify whether it could benefit diagnosis and treatment.
MATERIAL AND METHODS
Patients' characteristics
The study comprised 25 Iraqi cases treated in Baghdad Medical City (BMC), Iraq, 19 women and 6 men. All cases were examined histologically in the pathology department in BMC, Iraq, from 2020 to 2021. Twenty- five patients with thyroid carcinoma were diagnosed. Nine patients out of 25 were diagnosed with papillary thyroid carcinoma (PTC); in addition to 10 were diagnosed with follicular carcinoma (F.C.), 6 patients were diagnosed with follicular adenoma (F.A.).
Immunohistochemically study
Immunohistochemical anti-heat shock protein antibody and Expose HRP/DAB detection IHC (mouse and rabbit) kits were supplemented by Abcam Company. U.K. Senior pathologist verified the tissue specimens to include T.C. tissue for the study group to deal with the immunohistochemical study. Ten percent formalin was used for 24 hours to fix all tissue samples, followed by treatment of the samples with 70% ethanol overnight. Dehydration of the sample was achieved using an increased graded series of alcohol (70%, 90% and 100% ethanol) for two hours for each concentration. All samples were treated with xylene for 2 hours to replace the ethanol (clearing). Melted paraffin (at 58 0C) was used for tissue embedding for 2-3 hours at 60 0C in an oven and cut into sections by a microtome for 5 µm thickness, then floated on the water bath at 50°C then transferred onto a Superfrost Plus slides for next steps. Xylene was used to remove the paraffin from the slide in three steps for 10 minutes each. According to the procedure, endogenous peroxidase activity has to be blocked. Hydrogen peroxide, three percent, was used in 100% methanol for 25 minutes. All sections were washed with triethanolamine-buffered saline (TBS) at pH 7.6 after that. Citrate buffer (pH:6) was used to submerge the sections used to submerge the sections to activate the antigen retrieving and unmasking. Ten minutes was when all samples were put in a microwave. TBS at pH 7.6 was used to rinse the sections. All nonspecific reactions must be blocked; for this, all sections are incubated in 20% swine serum (Sigma, Aldrich, Germany) for 20 min. All sections were covered with antibody (primary) (at dilution 1/150 for HSP70) and incubated for 1 hour in a humidity chamber at 370C, followed by rinsing the sections gently in TBS. At room temperature, a secondary antibody was added for 10 Followed by washing for 10 minutes. Streptavidine-HRP antibody was added after that and Then washed to visualize the peroxide reaction. After that, hematoxylin was used to counterstain the slides for 30 seconds. Tap water was used to wash all slides, followed by dehydration. The slides were mounted with a permanent-mounting medium (DPX). The slides were examined under the optical microscope at 10 X and then at 40X magnifications. The above work steps were followed according to Basim et al., 202016.
Immunohistochemical evaluation
An experienced head and neck pathologist verified the immunohistochemistry sections. The scoring method used in this work was according to. If the average fraction of intensely staining cells occupied more than 25% of the total slide, it will be considered positive for HSP7017.
RESULTS AND DISCUSSION
Patients' characteristics
The study comprised 25 Iraqi patients treated in Baghdad Medical City (BMC), Iraq. Nineteen female and 6 male patients of average age of 44 years at surgery (range: 25–68 years) (Figure 1). According to the gender of patients who participated in this study, all three types of thyroid cancer were detected in female patients mainly. This may belong to certain reproductive factors, especially regarding estrogen receptor status18. In terms of age, patients between 20 and 29 years old (median age at diagnosis is 24.5), followed by 40 to 49 years old with a median of 44.5 years, had the higher incidence of PTC.
Regarding F.C. patients, the group of 60-69 years old had the highest incidence. However, the 50-59-year-old patients had the highest incidence of F.A. and the second-highest incidence of F.C. (Figure 2). Follicular adenoma appeared to be the chief type in the 50-59 years group. These results might be due to the aggressive disease and the usual decline of the immune response, especially in the 60-69-year-old group. Also, the post- menopausal hormonal changes in females might play an essential role in these incidence 19.
Immunohistochemistry
One of the main points in cancer pathology is understanding the mechanisms that belong to the origin of the loss of cell differentiation. Many works pointed to the role of molecular chaperones in maintaining cellular and tissue homeostasis 20,21. It is well known that an imbalance between cell proliferation and differentiation is crucial to ensure the correct growth and development of organisms and to maintain adult tissue and organ homeostasis. The HSP70- was determined as a present or absent antibody in the tissues. Digital camera, light m icr scope, and imaging computer software were used for histological analysis. Two criteria were used for the evaluation: intensity and localization of immunostaining in cells (nuclear or cytoplasmic). According to Table 1, it was 9 papillary thyroid carcinomas (PTC) cases, 10 follicular carcinoma (F.C.) and 6 follicular adenomas (F.A.) cases out of 25 T.C. patients. HSP70 protein was determined in 8 out of 10 (PTC) (Figure 3 A, B), all (F.C.) patients (10 cases) (Figure 3 C, D) and in all (F.A.) patients (six patients) (Figure 3 E, F).
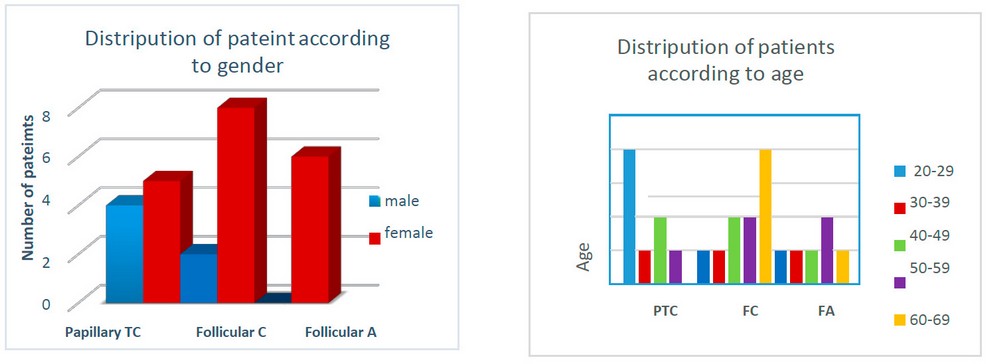
Figure 1. Distribution of patients according to gender. Figure 2. Distribution of patients according to age.
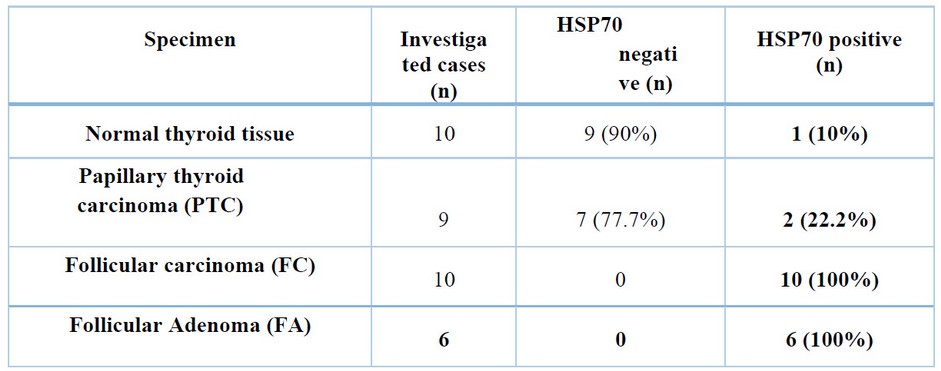
Table 1. Expression of HSP70 between different types of thyroid cancer. The number of investigated cases and the positive and negative HSP70 percentages are shown.
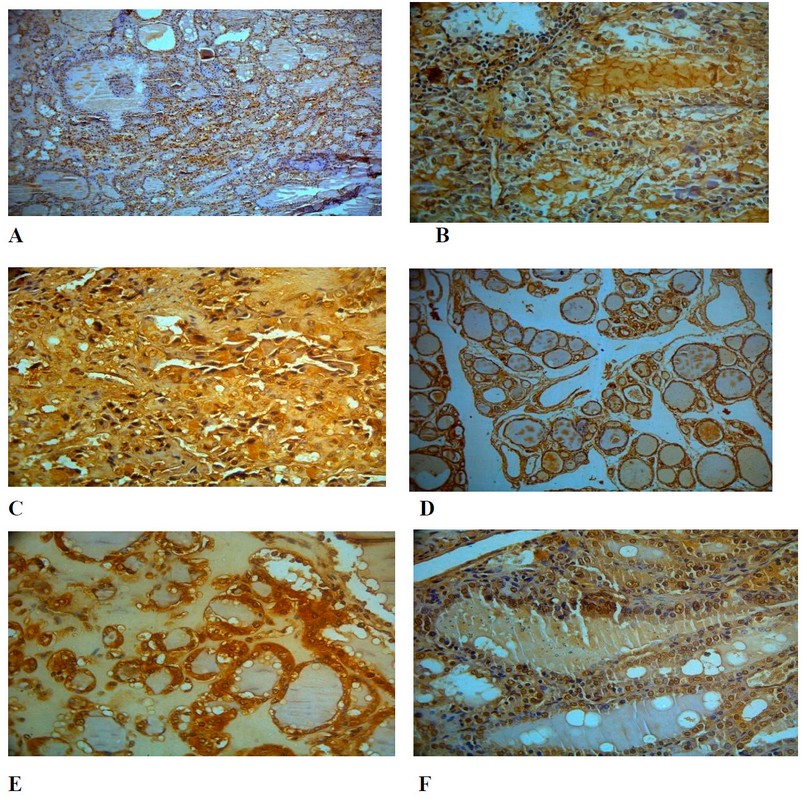

Figure 2. HSP70 immunostaining in different thyroid cancer cases 10 X. HSP70 was determined as a present or absent antibody in the tissues. A & B; PTC, C & D; FC; E&F; FA.
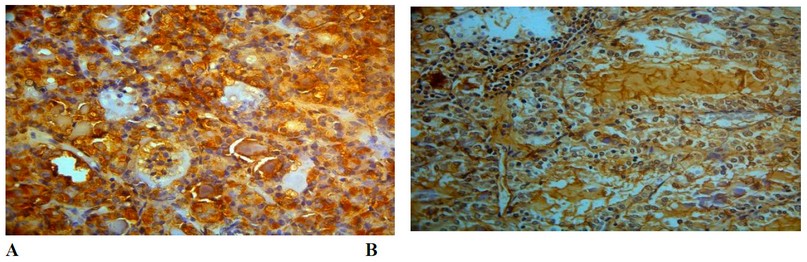
Figure 3. Expression of Hsp70 protein in thyroid cancer, Immunohistochemical analysis, (A) cytoplasmic localization, X40
(B) nuclear-cytoplasmic localization, X100.
On the other hand, a different localization of HSP70 has been detected. It has been pointed in nuclei of F.A. (Figure 2; F) and in the cytoplasm of PTC and F.A. (Figure 3; A, B), which consist of the results 22. Hsp70 protein has different localization in tumor cells23. These results might be due to differences in the stages of the disease, which means the correlation of HSP70 protein with the prognosis of the disease.
CONCLUSIONS
Molecular chaperones play a crucial role in maintaining cellular and tissue homeostasis. An increase in HSP70 levels occurs in thyroid cancer types (PTC, F.C., and F.A.). This raise can be exploited to detect the tumor's grade and predict the patient's response to the treatment. This can help to make a more accurate diagnosis and, finally, to achieve a more successful treatment. In addition, to avoid the harmful side effects of many drugs that might not work with certain types of thyroid cancer. Thus, physicians can reduce the time required to treat this type of cancer.
Funding
This research received no external funding.
Conflict of Interest
The authors declare that they have no conflicts of interest.
REFERENCIAS
1. Seib, C. D., & Sosa, J. A. Evolving Understanding of the Epidemiology of Thyroid Cancer. Endocrinology and Metabolism Clinics of NA. 2018.
2. Kitahara, Cari M., and Julie A. Sosa. Understanding the Ever-Changing Incidence of Thyroid Cancer. Nature Reviews Endocrinology, Springer US, 2020; (16): 617–618
3. Prete, Alessandro, et al. Update on Fundamental Mechanisms of Thyroid Cancer. 2020; (March), 1–10.
4. Liu, Y., Su, L., & Xiao, H. Review of Factors Related to the Thyroid Cancer Epidemic. International Journal of Endocrinology 2017; 1-9.
5. Zarebczan, B., and H. Chen. Multi-Targeted Approach in the Treatment of Thyroid Cancer. Minerva Chirurgica, 2010; 65 (1): 59–69.
6. Patel, Kepal N., and Ashok R. Shaha.Poorly Differentiated and Anaplastic Thyroid Cancer. Cancer Control, 2006; 13 (2): 119–28.
7. Rashmi, Ramachandran, and Santhosh Kumar. Ectopic Expression of Hsp70 Confers Resistance and Silencing Its Expression Sensitizes Human Colon Cancer Cells to Curcumin-Induced Apoptosis. Carcinogenesis 2004; 25 (2):179–87.
8. Avdalyan, A. M., et al. The Relationship of Immunoexpression of Ki-67 and Hsp70 with Clinical and Morphological Parameters and Prognosis of Papillary Thyroid Cancer. Bulletin of Experimental Biology and Medicine 2020; 168 (5): 688–93.
9. Soleimani, Atena, et al.Therapeutic Potency of Heat-Shock Protein-70 in the Pathogenesis of Colorectal Cancer: Current Status and Perspectives. Biochemistry and Cell Biology 2019; 97 (2): 85–90.
10. Blagosklonny, Mikhail V. Re: Role of the Heat Shock Response and Molecular Chaperones in Oncogenesis and Cell Death. Journal of the National Cancer Institute, 2001; 93 (3): 239–40.
11. Murphy, Maureen E. The HSP70 Family and Cancer. Carcinogenesis, 2013; 34 (6):1181–88.
12. Yaglom, Julia A., et al. High Levels of Heat Shock Protein HSP72 in Cancer Cells Suppress Default Senescence Pathways. Cancer Research, 2007; 67 (5): 2373–81.
13. Nylandsted, Jesper, et al. Heat Shock Protein 70 Promotes Cell Survival by Inhibiting Lysosomal Membrane Permeabilization. Journal of Experimental Medicine, 2004; 200(4): 425–35.
14. Daugaard, Mads, et al. Lens Epithelium-Derived Growth Factor Is an Hsp70-2 Regulated Guardian of Lysosomal Stability in Human Cancer. Cancer Research. 2007; 67 (6): 2559–67.
15. Juhasz, K., Lipp, A., Nimmervoll, B., Sonnleitner, A., Hesse, J., Haselgruebler, T., & Balogi, Z. The Complex Function of Hsp70 in Metastatic Cancer.Cancers. 2014; (6) 42–66.
16. Basim M. Khashman , Suhad K. Karim2 and Ghada Nazar Al-Jussani The oncogenic effect of EBV/HPV co-infection in a group of Iraqi women with cervical carcinoma. Biochemical and Cellular Archives. 2020; 20 (2): 6037–6040.
17. Syrigos, K. N., Harrington, K. J., Karayiannakis, A. J., Sekara, E., Chatziyianni, E., Syrigou, E. I., & Waxman, J.. Clinical significance of heat shock protein-70 expression in bladder cancer. Urology. 2003; 61(3), 677–680.
18. Liu, J., Xu, T., Ma, L., & Chang, W. Signal pathway of Estrogen and Estrogen Receptor in the development of Thyroid Cancer. Frontiers in Oncology 2021; Vol. 11. Frontiers media S.A. https://doi.org/10.3389/fonc.2021.593479)
19. MEGAN R. HAYMART. Understanding the Relationship Between Age and Thyroid Cancer. The Oncologist. 2009; 14:216 –221.
20. Walsh, D.; Grantham, J.; Zhu, X.O.; Lin, J.W.; Van Oosterum, M. Taylor, R.; Edwards, M. The role of heat shock proteins in mammalian differentiation and development. 1999; Environ. Med. 43, 79–87.
21. Barna, J.; Csermely, P.; Vellai, T. Roles of heat shock factor 1 beyond the heat shock response. Cell. Mol. Life Sci. 2018; 75, 2897–2916.
22. Avdalyan, A. M., Ivanov, A. A., Lushnikova, E. L., Molodykh, O. P., & Vikhlyanov, I. V. The Relationship of Immunoexpression of Ki-67 and Hsp70 with Clinical and Morphological Parameters and Prognosis of Papillary Thyroid Cancer. Bulletin of Experimental Biology and Medicine 2020; 168 (5): 688–693.
23. Malusecka E, Zborek A, Krzyzowska-Gruca S, Krawczyk Z. Immunohistochemical detection of the inducible heat shock protein Hsp70: A methodological study. J. Histochem. Cytochem. 2006; 54(2):183-190.
24. A. M. Avdalyan, A. A. Ivanov, E. L. Lushnikova, O. P. Molodykh, and I. V. Vikhlyanov. The Relationship of Immunoexpression of Ki-67 and Hsp70 with Clinical and Morphological Parameters and Prognosis of Papillary Thyroid Cancer; Bulletin of Experimental Biology and Medicine. 2020; Vol. 168, No. 5, ONCOLOGY.
Received: 28 September 2023/ Accepted: 15 November 2023 / Published:15 December 2023
Citation: Hassan H. A., Ali S. M., Al-hijazi A. Y.l. Joda A E. Expression of Heat Shock Protein 70 in Thyroid Gland Cancer. Revis Bionatura 2023;8 (4) 89. http://dx.doi.org/10.21931/RB/2023.08.04.89
Peer review information. Bionatura thanks anonymous reviewer(s) for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
All articles published by Bionatura Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Bionatura ISSN. 13909355. Scopus coverage years: from 2016 to the Present
Publisher's Note: Bionatura stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2023 by the authors. They were submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).






