CS 2019.02.01.17
Files > Conference Series > 2019 > Humboldt Kolleg 2019
Bionatura Conference Series Vol 2. No 1. 2019
“Breaking Paradigms: Towards a Multi-, Inter- and Transdisciplinary Science” In commemoration of the 250th Anniversary of Alexander von Humboldt
Alzheimer’s disease: An overview of the current treatments
Carolina Serrano-Larrea, David Clavijo-Calderón
available in: http://dx.doi.org/10.21931/RB/CS/2019.02.01.17
ABSTRACT
Alzheimer’s disease (AD) affects millions of people around the world and although there are treatments that help control symptoms and slow down the progress of the disease, there is still no cure. Current treatments include three acetylcholine inhibitors, a glutamate inhibitor and a combination of the two. Due to the failure of hundreds of clinical trials with monotherapies, multitarget treatments are currently being investigated that consider both brain and peripheral factors. Gene therapy is one of the most promising therapies to treat and prevent the development of AD. Nowadays, there is no available medical treatment based on gene therapy to treat AD; however, there are treatments in phase 1 and phase 2 clinical trials with promising results. In this review, we will focus on the most important gene therapy treatments, CERE-110 (adeno-associated virus AAV2-Nerve Growth Factor), Intracerebral AAV gene delivery of APOE2 and gene therapy using PPARγ-coactivator-1α(PGC-1α)
Keywords: Alzheimer’s disease, gene therapy, adeno-associated virus, lentiviral vectors, clinical trials.
INTRODUCTION
Population aging is the principal factor that relates to the increase in the global burden of Alzheimer disease (AD). The lack of trustworthy biomarkers and preventive strategies and treatments are the major challenges scientists face nowadays1. Current findings have changed the research pathway science is following for the cure of AD. The systemic disturbances separated from the brain degeneration could be silent processes related to the progression of the disease and not only secondary effects of dementia1.
Alzheimer Disease Mechanism
There are many factors that support the diagnostics of AD, the most prevalent are neuron loss, brain atrophy, synaptic pathology, and neuroinflammation. However, the hallmarks of AD commonly known are amyloid- β (Aβ) plaques and neurofibrillary tangles (NFTs).2
There are different risk factors that can lead to the development of AD, they are divided into genetic and non-genetic factors. Early-onset Alzheimer Disease (EOAD) is inherited by an autosomal dominant way and there have been identified three genes that cause mutations that trigger the disease, the amyloid precursor protein (APP), presenilin 1 (PS1), and presenilin 2 (PS2). 2 The only confirmed risk factor to develop late-onset Alzheimer’s disease (LOAD) is the APOE4 allele of the apolipoprotein E gene (ApoE) 2. There are other characteristics that can mean a risk of AD like Down Syndrome because of the trisomy in chromosome 21, which includes the gene for APP. Overall, age is the most influential factor for the development of AD and the risk increases exponentially over the 65 years.2
APP is present on the synapses of neurons and shows beneficial effects on growing and overall health of cells.4 Amyloid β comes from the proteolysis of APP by the action of α-secretase and γ-secretase. Aβ is present not only in the brain but also in some peripheral organs and tissues such as kidney, heart, liver, spleen, muscles, and others.3 Lower levels of Aβ have been reported in healthy individuals. There are remarkable differences between brain and peripheral Aβ aggregates. The most aggregation susceptible form of Aβ is Aβ42 and it is present mostly in the brain, it is also the most toxic. The other form, Aβ40 is mostly present on the peripheral tissues and the mechanism that underlies this difference is not well known4.
The Aβ42 degradation methods failure is an important cause to develop sporadic AD which holds most of the cases (99%). There are many ways the brain gets rid of Aβ, some of them include phagocytosis, macropinocytosis, and endocytosis those processes are performed by several brain cells, many enzymes and efflux to the peripheral circulation. The exact mechanism of clearing Aβ from the brain to the periphery is not clearly understood. Peripheral phagocytes, astrocytes, oligodendrocytes monocytes, microglia, and neurons have important roles in Aβ clearance.1
Cerebral amyloid angiopathy is another characteristic of AD in which amyloid aggregates in the walls of blood vessels of the CNS, this feature is related with ApoE ε4 allele. Otherwise, Glial cells have been found surrounding Aβ plaques which means they trigger the immune system and stars a neuroinflammation process that in many cases is beneficial but in chronic circumstances may result in accelerated neurodegeneration. Understanding the biological mechanism of degradation of Aβ is a key aspect in the development of AD drugs.2
Available treatment for Alzheimer Disease
The medications approved by the FDA for the treatment of AD are just five, and three of them are cholinesterase inhibitors, Donepezil, Galantamine and Rivastigmine. Those inhibitors avoid the elimination of acetylcholine (Ach) that is a key neurotransmitter for developing new memories, learning process, judgment, and other important processes. Constant levels of cholinesterase are important to maintain and compensate for the decrease in healthy neurons and maintain synaptic activity.5 Memantine is the fourth drug approved to treat AD by the FDA but only for moderate to severe stages. It performs uncompetitive antagonistic action over NMDA receptors and inhibits glutamate transmission.6 Cholinesterase inhibitors do not stop AD progression they work as a symptomatic treatment that inhibits the hydrolysis of Ach thus increasing its bioavailability to enhance communication between neurons in the cholinergic pathways and slow the development of AD symptoms.5
Nevertheless, current treatments and prevention mechanism for AD are not effective to cure the disease because there is not one treatment to fight one target, there is a huge complexity of AD mechanisms that must be considered.1 Over 100 clinical trials of monotherapies that target Aβ have failed so there is a clear need for multitarget therapies that consider the complex pathogenic machinery. There are many systemic disorders associated with AD such as cardiovascular failure, diabetes mellitus and metabolic diseases that are not the target of AD treatments so a therapy that focus on more targets than just the brain will be more effective.3
Figure 1. General views of actual treatments for Alzheimer Disease
Gene therapy systems as a new alternative to find a cure to AD
Gene therapy consists of introducing specific genes into the patient's cells to treat or prevent certain diseases. Here we classify gene therapy treatments for AD based on its vector: adeno-associated virus (AAV) or lentiviral vectors (LVs).
Adeno-associated virus vector
During the last years, the research for the treatments of neurodegenerative diseases has been bet by the use of the gene therapy and most of the research projects are based on the use of recombinant adeno-associated viral (rAAV) gene therapy with systemic or direct administration of the agents into the side of interest 7. rAAV are small, helper-dependent single-stranded DNA viruses and uses an exchange of nucleotide sequences to enable insertion, deletion or replacement of DNA sequences in cells 8. AAV vectors can transduce post-mitotic cells (neurons, astrocytes, and oligodendrocytes) with a cloning capacity of ~4.7 kb 9. AAV serotypes 1, 2, 5, 8, 9, and rhesus (rh).10 are the most used on the central nervous system and their specificity depends on the cell type and region of the brain, and animal species to be inserted 10.
One of the first treatments tested on humans was gene therapy of nerve growth factor (NGF) to reduce cholinergic cell loss in AD. NGF does not cross the blood brain barrier so CERE-110 (AAV2-NGF), is administered on the nucleus basalis of Meynert through stereotactic injections.11 Phase 1 clinical trial in subjects with mild to moderate AD to assess the safety, tolerability and biologic activity of in vivo AAV serotype 2-mediated delivery of CERE-110, implanting genetically modified fibroblasts to express human NGF into the forebrain. The studies began in 2004 and the clinical trial began in July 2014 and ended in October 2016 (Clinical trial NCT00087789).12 This is a Phase I clinical study with 10 subjects with mild to moderate AD. Finally, the results of phase 1 clinical trials shown that CERE-110 was safe and well-tolerated for up to two years of subjects analysis. However, on the Phase 2 trial in 49 subjects, the results show that CERE-110 was again safe and well-tolerated, but ineffective, giving the same results the subjects treated with the AAV2-NGF and patients with placebo.13
The APOE gene (chromosome 19, position 13.32) encodes for apolipoprotein E, which regulates lipid metabolism and redistribution on the bloodstream. In humans, the APOE gene presents three different isoforms: APOE2, APOE3, and APOE4. The common allele in the population is APOE3.14 There is some evidence that correlates the presence of a specific allele with the development of AD. APOE4 is highly involved in the development of AD, APOE3 is considered neutral in the development of AD, and APOE2 is considered a protective form and counteracts APOE4 in animal models.15 In addition, Intracerebral AAV gene delivery of APOE2 (AAVrh.10 and AAV9 vectors) presents promising results on the AD treatment. Studies presented by Zhao and collaborators show a reduction in brain soluble and insoluble Aβ levels as well as an amyloid burden in mouse models, the pathology is dependent on human APOE4. The efficacy of APOE2 was highly dependent on brain APOE2 levels and the amount of pre-existing Ab and amyloid deposition. A reduction of brain Aβ burden can be seen after a single injection, intrathalamic delivery, of vector AAV expressing APOE2 gene.16 To continue with the preclinical evaluation and to assess the CNS distribution and safety of APOE2 gene therapy for AD in animal models, Rosemberg and collaborators17 evaluated intraparenchymal, intracisternal, and intraventricular routes of delivery to the CNS of nonhuman primates of an AAVrh.10 serotype coding for an HA‐tagged human APOE2 cDNA sequence (AAVrh.10hAPOE2). The results show that the three routes result in the safe and effective expression of ApoE2‐HA in the regions affected in AD, being the intracisternal administration the preferred route due to the least invasive and widespread expression throughout the brain.17 Finally, after all, preclinical studies in animals, a clinical trial is held in 15 participants (NCT03634007) to treat patients who are APOE4 homozygotes diagnosed with dementia due to AD with the presence of amyloid plaques. The purpose of this study is to measure safety, toxicity and establish a maximum tolerable dose of intracisternal administration of AAVrh.10hAPOE2. The study will start in June 2019 and will end in December 2021.18
Lentiviral vectors
LVs are enveloped single-stranded RNA retroviruses that can infect both dividing and nondividing cells and are highly useful for gene therapy treatments of degenerative diseases, especially recombinant nonreplicative HIV-1–based LVs.10
One of the most promising methods using LVs is the application of gene therapy using PPARγ-coactivator-1α(PGC-1α), a study presented by Katsouri and collaborators19 in order to treat causes of AD in mice. PGC-1α has been implicated in various neurodegenerative processes, and patients with AD have shown decreasing expression of this cofactor. Absence of PGC-1α has been linked with the augmented level of Aβ peptides. Aβ is produced by β-APP cleaving enzyme (BACE1), whose transcription has been demonstrated to be regulated by cofactor PGC-1α. In relation to the above, some previous experiences have shown interesting results among PGC-1α presence and Aβ generation levels. Some of these experiences include:
Use of exogenous human PGC-1α expression in regions of mice brains, resulting in a decrease of Aβ. Use of siRNA transfection from mice with decrease or silence PGC-1α expression to normal mice, resulting in a proliferation of Aβ levels. Pharmacological stimulation of PGC-1α synthesis, resulting in decreasing Aβ concentration. Treatments with resveratrol, PGC-1α activator stimulated the activity of Aβ-degrading enzyme neprilysin and reduced the amyloid plaques.
For the current experiment, authors hypothesized that gene therapy developing PGC-1α to the brain can work as a neuroprotector acting over the cause of AD-related with Aβ production. The main idea is to create an LVs expressing PGC-1α, to do a stereotaxic delivery of human PGC-1α in transgenic mice and prove its effects as a neural protector from AD. In the first stage, they achieve very optimist results, since they can obtain effective transduction of PGC-1α using RVG-B2c LVs. In addition, they measured a high expression of hPGC-1α protein in mice injected with the LV. In a second stage, they tested the effects of PGC-1α developed, in mice with preclinical symptoms of AD. Results of this part conclude that gene delivery improved spatial and recognition memory in mice. In this stage, they also proved the effect of hPGC-1α related to Aβ production. The chronic expression of the protein demonstrated to be effective in reducing BACE1, Aβ levels, and plaque load in treated mice. hPGC-1α demonstrated to be useful in attenuating neuroinflammation in mice and preventing the neural loss in the hippocampus of treated mice.19
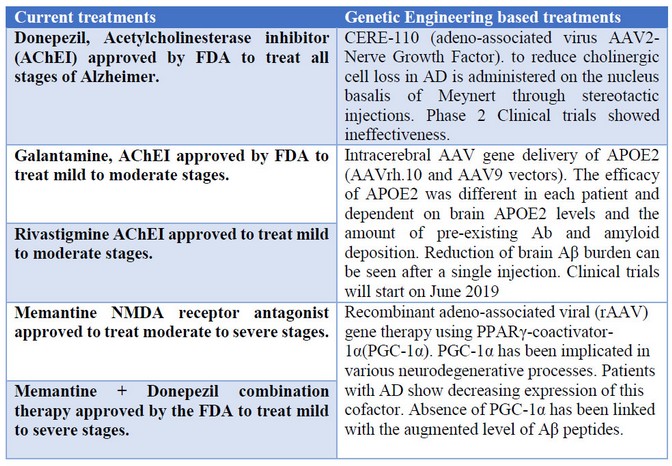
Table 1. Summary and comparison of current treatments and genetic engineering based treatments.
CONCLUSIONS
AD is the main form of dementia and besides being incurable, current treatments only focus on inhibiting the reuptake of acetylcholine or the action of glutamate and are administered depending on the stage of the disease. The mechanisms of action by which the Aβ forms the plaques or the formation of NFTs are not yet completely determined, however, the available information opens the doors to new therapeutic targets to treat the disease. AD is not only mental illness but also encompasses systemic factors that must be considered when designing effective treatments.
Gene therapy is a rising tool to treat AD. Although that gene therapy of nerve growth factor (NGF) reduce cholinergic cell loss, in AD does not give good results. APOE2 gene therapy and PGC-1α has promising results. Intracerebral AAV gene delivery of APOE2 after giving excellent results on reducing Aβ levels on animal models, a clinical trial with 15 patients diagnosed with dementia due to AD (APOE4 homozygotes) is performed to measure safety, toxicity and doses through intracisternal administration. Finally, PGC-1α show high qualities as a candidate for gene therapy actuating as a preserver of neural viability and improving the memory in AD, proving it has neuroprotector capabilities.
REFERENCES
1. Wang J, Gu BJ, Masters CL, Wang Y-J. A systemic view of Alzheimer disease — insights from amyloid-β metabolism beyond the brain. Nat Rev Neurol. 2017 Sep;13(10):612–23.
2. Crehan H, Lemere CA. Anti-Amyloid-β Immunotherapy for Alzheimer’s Disease. In: Developing Therapeutics for Alzheimer’s Disease. Elsevier; 2016. p. 193–226.
3. Bondi MW, Edmonds EC, Salmon DP. Alzheimer’s Disease: Past, Present, and Future. J Int Neuropsychol Soc. 2017 Oct;23(9–10):818–31.
4. O’Brien RJ, Wong PC. Amyloid Precursor Protein Processing and Alzheimer’s Disease. Annu Rev Neurosci. 2011 Jul;34(1):185–204.
5. Colovic MB, Krstic DZ, Lazarevic-Pasti TD, Bondzic AM, Vasic VM. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr Neuropharmacol. 2013 Apr;11(3):315–35.
6. Matsunaga S, Kishi T, Nomura I, Sakuma K, Okuya M, Ikuta T, et al. The efficacy and safety of memantine for the treatment of Alzheimer’s disease. Expert Opin Drug Saf. 2018 Oct;17(10):1053–61.
7. Hocquemiller M, Giersch L, Audrain M, Parker S, Cartier N. Adeno-Associated Virus-Based Gene Therapy for CNS Diseases. Hum Gene Ther [Internet]. 2016/06/07. 2016 Jul 1;27(7):478–96. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27267688
8. Combs B, Kneynsberg A, Kanaan NM. Gene Therapy Models of Alzheimer’s Disease and Other Dementias. Methods Mol Biol [Internet]. 2016;1382:339–66. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26611599
9. Terzi D, Zachariou V. Adeno-associated virus-mediated gene delivery approaches for the treatment of CNS disorders. Biotechnol J [Internet]. 2008 Dec;3(12):1555–63. Available from: http://doi.wiley.com/10.1002/biot.200800284
10. Piguet F, Alves S, Cartier N. Clinical Gene Therapy for Neurodegenerative Diseases: Past, Present, and Future. Hum Gene Ther [Internet]. 2017 Nov;28(11):988–1003. Available from: http://www.liebertpub.com/doi/10.1089/hum.2017.160
11. Tuszynski MH, Thal L, Pay M, Salmon DP, U HS, Bakay R, et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med [Internet]. 2005 May 24;11(5):551–5. Available from: http://www.nature.com/articles/nm1239
12. ClinicalTrials.gov. CERE-110 in Subjects With Mild to Moderate Alzheimer’s Disease [Internet]. Available from: https://clinicaltrials.gov/ct2/show/results/NCT00087789?term=gene+therapy&cond=Alzheimer+Disease&rank=4
13. ALZFORUM. Therapeutics CERE-110 [Internet]. Available from: https://www.alzforum.org/therapeutics/cere-110
14. Genetics Home Reference. APOE gene. Available from: https://ghr.nlm.nih.gov/gene/APOE
15. Fan S. The Gene Therapy Trial Aiming to Fend Off Alzheimer’s [Internet]. SingularityHub. 2019. Available from: https://singularityhub.com/2019/03/05/the-gene-therapy-trial-aiming-to-fend-off-alzheimers/
16. Zhao L, Gottesdiener AJ, Parmar M, Li M, Kaminsky SM, Chiuchiolo MJ, et al. Intracerebral adeno-associated virus gene delivery of apolipoprotein E2 markedly reduces brain amyloid pathology in Alzheimer’s disease mouse models. Neurobiol Aging [Internet]. 2016 Aug;44:159–72. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0197458016300574
17. Rosenberg JB, Kaplitt MG, De BP, Chen A, Flagiello T, Salami C, et al. AAVrh.10-Mediated APOE2 Central Nervous System Gene Therapy for APOE4-Associated Alzheimer’s Disease. Hum Gene Ther Clin Dev [Internet]. 2018 Mar;29(1):24–47. Available from: http://www.liebertpub.com/doi/10.1089/humc.2017.231
18. ClinicalTrials.gov. Gene Therapy for APOE4 Homozygote of Alzheimer’s Disease [Internet]. Available from: https://clinicaltrials.gov/ct2/show/NCT03634007
19. Katsouri L, Lim YM, Blondrath K, Eleftheriadou I, Lombardero L, Birch AM, et al. PPARγ-coactivator-1α gene transfer reduces neuronal loss and amyloid-β generation by reducing β-secretase in an Alzheimer’s disease model. Proc Natl Acad Sci [Internet]. 2016 Oct 25;113(43):12292–7. Available from: http://www.pnas.org/lookup/doi/10.1073/pnas.1606171113
Received: 10 April, 2019
Accepted: 25 May 2019
Carolina Serrano-Larrea, David Clavijo-Calderón
School of Biological Sciences and Engineering, Yachay Tech University
Corresponding author: [email protected]
