CS 2019.02.01.25
Files > Conference Series > 2019 > Humboldt Kolleg 2019
Bionatura Conference Series Vol 2. No 1. 2019
“Breaking Paradigms: Towards a Multi-, Inter- and Transdisciplinary Science” In commemoration of the 250th Anniversary of Alexander von Humboldt
Situation of Human Papilloma Virus: Generalities and current treatments
Cristhian Preciado and Paolo Vallejo Janeta
available in: http://dx.doi.org/10.21931/RB/CS/2019.02.01.25
ABSTRACT
Human papillomavirus represents the commonest STD, and it is the principal causative agent of different genital lesions, including genital warts and cervical cancer. Two high-risk HPV genotypes are responsible for the 70% of cervical cancer, and the prevalence of these types reaches alarming levels in many developing countries (e.g., 43.58% in Ecuador). However, the information about HPV in developing countries is limited, becoming an obstacle for breakthrough treatments or prevention strategies. Current strategies include the development of new vaccines, combined chemo- and radiotherapy, and the use of CRISPR/Cas9. Moreover, HPV can be used to treat other non-related cancers, such as ovarian cancer. The present review aims to describe the different prevention strategies and treatments for HPV available worldwide, cover the usage of HPV for the treatment of other diseases, as well as comment on their possible application in Ecuador.
Keywords: Human papillomavirus (HPV), treatments, CRISPR/Cas9
INTRODUCTION
Human papillomaviruses (HPV) constitute the most diverse group of the family Papillomaviridae, with more than 200 types described and sequenced1. The infection by HPV can lead to different diseases varying from genital warts to neoplasia and cervical & oropharyngeal carcinoma2–5. Among HPV, some genotypes are considered of high risk (including HPV 16, 18, 31, 52, and 58), which can trigger cancer development. While the remaining HPV types (“low-risk”) are typically associated with warts in the skin or mucosal zones (condyloma acuminata)6. The 70% of cervical cancer cases are related to HPV types 16 and 18, although they are not sufficient to cause for oncogenesis7,8. The last mentioned types are the primary targets for vaccine development to prevent infections and skin abnormalities7,9. Infection by HPV represents the most common sexually transmitted disease (STD) with their highest prevalence in women under 25 years old, and cervical cancer being one of the most common cancers in women7,9.
A study by Forman, et al. corroborates this fact, which also estimates the worldwide prevalence of HPV infections at 11.7% of women. However, this prevalence is heterogeneously distributed with developing regions being the most affected. For instance, the prevalence of South America reaches 15.3% among women compared with 4.7% in North America10. In Ecuador, the information regarding the prevalence and epidemiology of HPV is limited11–13. This prevalence varies according to the studied population. A study conducted in only cervical cancerous or precancerous lesions found an 86% of prevalence among Ecuadorian women, while a screening including cancerous and non-cancerous samples from Santa Elena, found 43.58% positive samples for HPV, values higher than Brazil and Peru11,13.
In Ecuador, HPV 16 and 18 remain with high prevalences in cervical cancer and another low/high-grade cervical lesions14,15, although other studies mention the emergence of different high-risk genotypes including HPV 58, 31, and 5211–14. Available data cover only cervical cancer and squamous cell carcinoma in women (Figures 1-2); there is no information accounting for cancer cases in men14. Treatment and prevention strategies for this specific geographic area include bivalent and tetravalent vaccines, which protect mainly against HPV 16 and 1813,14. Thereby, the effectiveness of such vaccines could be not enough in a country where deaths by cervical cancer occupy the second place in cancer deaths among women14.
The present review aims to describe the different prevention strategies and treatments for HPV available worldwide, cover the usage of HPV for the treatment of other diseases, as well as comment on their possible application in Ecuador.
Figure 1: Common types of HPV in cervical cancer cases (Data from Bruni, et.al., 2018)
Figure 2: Common types of HPV in squamous cell carcinomas cases (Data from Bruni, et.al., 2018)
Development of vaccines against HPV
Currently, prophylactic vaccines against HPV are applied in order to provide protection; these have proven to be highly efficacious in the generation of serum neutralizing antibodies against the virus16. Some of the treatments against HPV include the construction of quadrivalent and bivalent vaccines. The quadrivalent vaccine contains highly purified virus-like particles of the L1 protein. It is an adjuvanted non-infectious recombinant vaccine against HPV types 6, 11, 16, and 1817. While the bivalent vaccine includes oncogenic HPV types 16 and 18.
Up to now, these vaccines have included fusion proteins, protein along with adjuvants, encapsulated nucleotides, constructs of DNA, dendritic cells, and chimeric VLPs constructs. Tested in patients with end-stage cervical cancer, also in patients with intraepithelial neoplasia of the cervix, vulva, or perianal area and healthy patients18, these investigations proved the safety and limited immunogenicity of these vaccines. However, the evidence of T cell-induced responses is minimal. On the other hand, like other pharmaceutics, HPV vaccines are not entirely risk-free, and minor adverse effects could result from vaccination17,18.
Cisplatin or Radiation treatment against HPV
Several types of cancer, such as squamous cell cancer, are associated with HPV. Previous studies have demonstrated that advanced-stage HPV-associated cancers have a higher chance of cure than those with HPV-negative cancers19. Very effective treatments involve platinum-based chemotherapy (using cisplatin) and fractionated radiation therapy. Cisplatin reacts with guanine by forming intrachromosomal and interchromosomal bonds; this reaction causes irreversible damage in DNA, triggering cell death20.
Spanos et al.20 used this treatment against head and neck squamous cell cancer (HNSCC), on groups of mice where papillomavirus is associated and groups where it was not (HPV-positive and HPV-negative tumors). The results showed more sensitivity to combined cisplatin and radiation therapy in HPV-positive tumors compared with HPV-negative counterparts, and an intact immune response for tumor elimination was needed. Through the use of this combined technique, HPV-positive tumors were eradicated in almost half of the treated mice. However, large tumors required the addition of Ad-E6/E7 vaccine to approach elimination in 90% of mice. The conclusions of this outcome could serve as a model for novel therapies which improve tumor eradication for HPV-positive cancers.
Use of CRISPR/Cas9 for HPV treatment
The uncontrolled expression of human papilloma oncogenes E6 and E7 represent a high risk of cervical cancer development21. These oncoproteins perform pleiotropism in joining to host-cell proteins, aiming to tumor suppressor genes p53 and pRb as their primary targets. The effect of this interaction (proliferation, unrestricted division, and malignant transformation of infected cells) convert E6 and E7 oncogenes into ideal therapeutic targets22,23.
Zhen et al.21 designed a gene-specific therapy using a CRISPR/Cas9 system targeting promoter of HPV 16 E6/E7 and aiming their protein transcripts. This treatment model includes the transduction of the system into the cervical HPV-16-positive cell line SiHa. The technique causes an accumulation of p53 and p21 protein on CRISPR/Cas9 targeting promoter and on E6 and E7 oncogenes. Consequently, a decrease in proliferation activity on cervical tumor cells in vitro was observed21,23.
After transfection, tumor animal model is established by subcutaneous inoculation of cells into mice. As a result of this, the researchers observed inhibition in tumorigenesis, and those mice incubated with CRISPR/Cas9 targeting-transcript followed up their average growth. Thus, the application of CRISPR/Cas9 aiming at HR-HPV prime oncogenes constitutes a novel method to treat cervical cancer as much as its use in other different HPV-related cancer therapies21.
Novel strategies
Different techniques have been developed for the abrogation of either E6 or E7 oncogenes by targeting gene expression or protein-protein interaction24. Besides therapeutic nucleic acid strategies, antisense oligodeoxynucleotides (ASO) and ribozymes (Rz) targeted are used to inhibit the expression of HPV E6/ E7 oncogenes; performing some positive results, however, there are several limitations such as low efficiency, short-period maintenance, and high costs24,25.
In later research, Clawson et al.26 employed ASO targeted to access in the HPV11E6/E7 RNA sites, acquired using library selection protocols. This technique demonstrated its effectiveness in blocking the progression of HPV-induced papillomas in human foreskin grafts on immunodeficiency mice.
Another approach, is the infection of the HPV, expressing cervical carcinoma cell, with recombinant viruses expressing full-length E2 protein27. This E2 binds the early promoter of HPV and acts repressing the E6 and E7 oncogenes transcription, which will end in the maintenance of p53 and pRb reactivation28.
The inclusion of CIGB-300 constitutes a breakthrough in cervical cancer treatment. This peptide disrupts the phosphorylation process mediated by CK229,30, a kinase protein that enhances stem cell maintenance and cell proliferation in tumors31. CIGB-300 aims explicitly to the npm/B23 oncoprotein leading to apoptosis through the modulation of numerous cell processes as PCD, cell motility, and proliferation29. Concerning cervical carcinoma treatment, various clinical trials included the formulation of CIGB-300.
The first trial applied intracervical injections of increasing doses (14 to 490 mg) in women diagnosed with cervical cancer, yielding reductions in lesions of 75% of patients, full recession in 19% and elimination of HPV in half of the patients32. The effect of CIGB in reducing tumors was dose-dependent; however, other studies established the maximum tolerated dose (MTD) at 70 mg33. Further trials combined previous chemotherapeutic agents and CK2 inhibitors. For instance, the combination of CIGB-300 and cisplatin showed promissory results in reducing tumoral lesions31.
Use of HPV pseudovirions for treatment of other diseases.
Nowadays, researchers discovered a way to package DNA plasmids into the L1 and L2 capsid proteins of papillomavirus in order to create “pseudovirions” (PsV), these pseudovirions can be used to deliver DNA into different cell types and tissues34,36. The abilities performed by these PsVs in DNA delivery propose their application in basic biology, such characterization processes and neutralizing assays; and also their use into the medical field35. Up to now, this novel technique has been applied in gene therapy for the treatment of ovarian cancer using pseudovirions, which carry a gene containing the herpes simplex virus thymidine kinase (HSV-tk) (Figure 3), revealing positive results36.
The advantages of pseudovirion technologies aim to the generation of broad applications in direct medicine, cancer therapy, and the treatment of many autoimmune diseases. Another approach is the design of protocols in order to achieve mass production of pseudovirions, which can be used for vaccination in massive amounts.
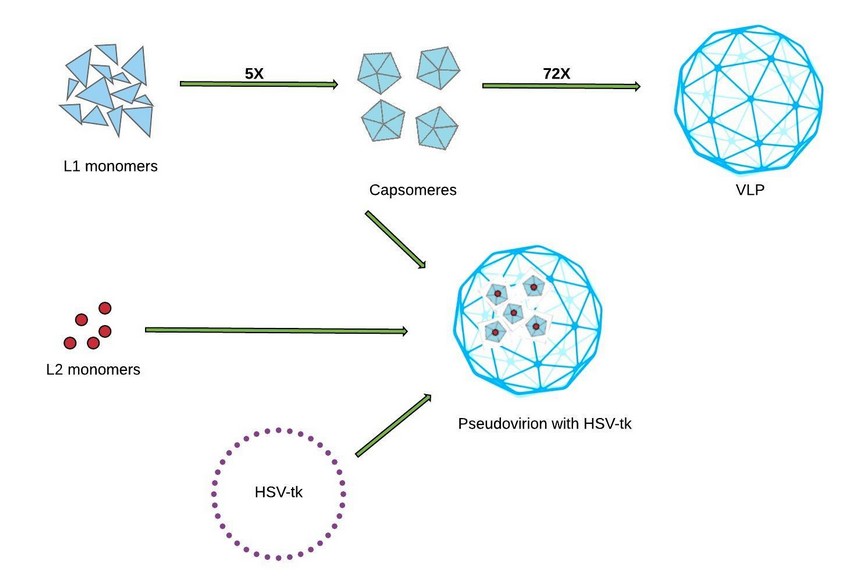
Figure 3: Process to develop the HPV pseudovirions containing HSV-tk (Adapted from Hung, et.al., 2012)
CONCLUSIONS
Currently, many approaches face the problem of HPV infection and HPV-related diseases. The prophylactic method includes the application of bivalent and quadrivalent vaccines which protect against the commonest HPV-cancer related types. These vaccines and improvements performed in them have shown safety and effectiveness in healthy and cancer patients. However, protection against more HPV types should be considered, especially in countries (like Ecuador) where other genotypes have a higher prevalence and where cancer statistics are very high.
Regarding the treatment of HPV-related cancer, chemotherapy using cisplatin combined with radiation seems to be an adequate model to eliminate small and large tumor (with Ad-E6/E7 vaccines). Furthermore, CRISPR/Cas9 targeting E6/E7 HPV oncogenes to produce an accumulation of p53 and pRb tumor suppressor genes also showed promising results in animal essays. ASO and Rz also showed significant results in blocking the progression of papillomas and repression of E6/E7 oncogenes. CIGB-300 is also a novel strategy to disrupt the growth, and even eliminate, tumoral lesions in cervical cancer patients, showing excellent results working with other chemotherapeutic agents.
Taking into consideration the legal and technological implications from developing countries, the treatment using cisplatin, radiation, and vaccines could be an adequate approach to reduce the prevalence in such countries.
Finally, HPV is not only a target for viral treatments. This virus is also used as treatment against other cancer types. For instance, the package of HSV-tk in HPV pseudovirions showed positive results in the treatment of ovarian cancer.
REFERENCES
1. PaVE: Papillomavirus Episteme. Reference genomes for Human papillomavirus [Internet]. Human Genomes. 2017 [cited 2019 May 15].
2. Alemany L, Saunier M, Alvarado-Cabrero I, Quirós B, Salmeron J, Shin H-R, et al. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide: HPV in Anal Cancers. Int J Cancer. 2015 Jan 1;136(1):98–107.
3. Pannone G, Santoro A, Papagerakis S, Lo Muzio L, De Rosa G, Bufo P. The role of human papillomavirus in the pathogenesis of head & neck squamous cell carcinoma: an overview. Infect Agent Cancer. 2011 Mar 29;6(1):4.
4. Egawa N, Egawa K, Griffin H, Doorbar J. Human Papillomaviruses; Epithelial Tropisms, and the Development of Neoplasia. Viruses. 2015 Jul;7(7):3863–90.
5. Forcier M, Musacchio N. An overview of human papillomavirus infection for the dermatologist: disease, diagnosis, management, and prevention. Dermatol Ther. 2010;23(5):458–76.
6. Cutts FT, Franceschi S, Goldie S, Castellsague X, Sanjose S de, Garnett G, et al. Human papillomavirus and HPV vaccines: a review. Bull World Health Organ. 2007 Sep;85:719–26.
7. Woodman CBJ, Collins SI, Young LS. The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer. 2007 Jan;7(1):11–22.
8. Muñoz N, Castellsagué X, de González AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006 Aug 21;24:S1–10.
9. Garland SM, Kjaer SK, Muñoz N, Block SL, Brown DR, DiNubile MJ, et al. Impact and Effectiveness of the Quadrivalent Human Papillomavirus Vaccine: A Systematic Review of 10 Years of Real-world Experience. Clin Infect Dis. 2016 Aug 15;63(4):519–27.
10. Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global Burden of Human Papillomavirus and Related Diseases. Vaccine. 2012 Nov;30:F12–23.
11. Brown CR, Leon ML, Muñoz K, Fagioni A, Amador LG, Frain B, et al. Human papillomavirus infection and its association with cervical dysplasia in Ecuadorian women attending a private cancer screening clinic. Braz J Med Biol Res. 2009 Jul;42(7):629–36.
12. García Muentes GD, García Rodríguez LK, Burgos Galarraga RI, Almeida Carpio F, Ruiz Cabezas JC. Genotypes distribution of human papillomavirus in cervical samples of Ecuadorian women. Rev Bras Epidemiol. 2016 Mar;19:160–6.
13. Mejía L, Muñoz D, Trueba G, Tinoco L, Zapata S. Prevalence of human papillomavirus types in cervical cancerous and precancerous lesions of Ecuadorian women. J Med Virol. 2016;88(1):144–52.
14. Bruni L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J, et al. Human Papillomavirus and Related Diseases in Ecuador [Internet]. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre); 2018 Dec [cited 2019 May 14] p. 74. Available from: https://hpvcentre.net/statistics/reports/ECU.pdf
15. Dalgo Aguilar P, Loján González C, Córdova Rodríguez A, Acurio Páez K, Arévalo AP, Bobokova J. Prevalence of High-Risk Genotypes of Human Papillomavirus: Women Diagnosed with Premalignant and Malignant Pap Smear Tests in Southern Ecuador [Internet]. Infectious Diseases in Obstetrics and Gynecology. 2017 [cited 2019 May 16]. Available from: https://www.hindawi.com/journals/idog/2017/8572065/abs/
16. Stanley, M. (2008). Immunobiology of HPV and HPV vaccines. Gynecologic Oncology, 109(2), S15–S21.doi:10.1016/j.ygyno.2008.02.003
17. Stillo, M., Carrillo Santisteve, P., & Lopalco, P. L. (2015). Safety of human papillomavirus vaccines: a review. Expert Opinion on Drug Safety, 14(5), 697–712.doi:10.1517/14740338.2015.1013532
18. Trimble, C. L., & Frazer, I. H. (2009). Development of therapeutic HPV vaccines. The Lancet Oncology, 10(10), 975–980.doi:10.1016/s1470-2045(09)70227-x
19. Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus–positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261-269.
20. Spanos, W. C., Nowicki, P., Lee, D. W., Hoover, A., Hostager, B., Gupta, A., … Lee, J. H. (2009). Immune Response During Therapy With Cisplatin or Radiation for Human Papillomavirus–Related Head and Neck Cancer. Archives of Otolaryngology–Head & Neck Surgery, 135(11), 1137. doi:10.1001/archoto.2009.159
21. Zhen, S., Hua, L., Takahashi, Y., Narita, S., Liu, Y.-H., & Li, Y. (2014). In vitro and in vivo growth suppression of human papillomavirus 16-positive cervical cancer cells by CRISPR/Cas9. Biochemical and Biophysical Research Communications, 450(4), 1422–1426.doi:10.1016/j.bbrc.2014.07.014
22. Boulet, G., Horvath, C., Broeck, D. V., Sahebali, S., & Bogers, J. (2007). Human papillomavirus: E6 and E7 oncogenes. The International Journal of Biochemistry & Cell Biology, 39(11), 2006–2011.doi:10.1016/j.biocel.2007.07.004
23. Doorbar, J., (2006). Molecular biology of human papillomavirus infection and cervical cancer. Clinical Science, 110(5), 525–541. doi:10.1042/cs20050369
24. Stanley, M. A. (2002). Prognostic factors and new therapeutic approaches to cervical cancer. Virus Research, 89(2), 241–248.doi:10.1016/s0168-1702(02)00192-2
25. Snoeck, R. (2006). Papillomavirus and treatment. Antiviral Research, 71(2-3), 181–191. doi:10.1016/j.antiviral.2006.06.007
26. Clawson, G.A., Miranda, G.Q., Sivarajah, A., Xin, P., Pan, W., Thiboutot, D., Christensen, N.D., 2004. Inhibition of papilloma progression by antisense oligonucleotides targeted to HPV11 E6/E7 RNA. Gene Ther. 11, 1331–1341
27. Goodwin, E.C., DiMaio, D., 2000. Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways. Proc. Natl. Acad. Sci. U.S.A 97, 12513–12518.
28. Wells, S.I., Francis, D.A., Karpova, A.Y., Dowhanick, J.J., Benson, J.D., Howley, P.M., 2000. Papillomavirus E2 induces senescence in HPV-positive cells via pRB- and p21(CIP)-dependent pathways. EMBO J. 19, 5762–5771.
29. Perea SE, Perera Y, Baladrón I, González L, Benavent F, Fariña HG, García I, Rodríguez A, Reyes V, García Y, Gómez R. CIGB-300: A Promising Anti-Casein Kinase 2 (CK2) Peptide for Cancer Targeted Therapy. Protein Kinase CK2 Cellular Function in Normal and Disease States 2015 (pp. 281-298). Springer, Cham.
30. Perera Y, Farina HG, Gil J, Rodriguez A, Benavent F, Castellanos L, Gómez RE, Acevedo BE, Alonso DF, Perea SE. Anticancer peptide CIGB-300 binds to nucleophosmin/B23, impairs its CK2-mediated phosphorylation, and leads to apoptosis through its nucleolar disassembly activity. Molecular cancer therapeutics. 2009 May 1;8(5):1189-96.
31. Lian H, Su M, Zhu Y, Zhou Y, Soomro SH, Fu H. Protein Kinase CK2, a Potential Therapeutic Target in Carcinoma Management. Asian Pacific journal of cancer prevention: APJCP. 2019 Jan 25;20(1):23-32.
32. Perea SE, Reyes O, Baladron I, Perera Y, Farina H, Gil J, Rodriguez A, Bacardi D, Marcelo JL, Cosme K, Cruz M. CIGB-300, a novel proapoptotic peptide that impairs the CK2 phosphorylation and exhibits anticancer properties both in vitro and in vivo. Molecular and cellular biochemistry. 2008 Sep 1;316(1-2):163-7.
33. Perea SE, Baladron I, Valenzuela C, Perera Y. CIGB-300: A peptide-based drug that impairs the Protein Kinase CK2-mediated phosphorylation. Seminars in oncology 2018 Jan 1 (Vol. 45, No. 1-2, pp. 58-67). WB Saunders.
34. Ma, B., Roden, R. B., Hung, C.-F., & Wu, T.-C. (2011). HPV pseudovirions as DNA delivery vehicles. Therapeutic Delivery, 2(4), 427–430. doi:10.4155/tde.11.28
35. Buck, C. B., Pastrana, D. V., Lowy, D. R., & Schiller, J. T. (n.d.). Generation of HPV Pseudovirions Using Transfection and Their Use in Neutralization Assays. Human Papillomaviruses, 445–462. doi:10.1385/1-59259-982-6:445
36. Hung, C.-F., Chiang, A. J., Tsai, H.-H., Pomper, M. G., Kang, T. H., Roden, R. R., & Wu, T.-C. (2012). Ovarian Cancer Gene Therapy Using HPV-16 Pseudovirion Carrying the HSV-tk Gene. PLoS ONE, 7(7), e40983. doi:10.1371/journal.pone.0040983
Received: 1 April 2019
Accepted: 2 May 2019
Cristhian Preciado - [email protected]
Paolo Vallejo Janeta - [email protected]
Genetic Engineering, School of Biological Sciences and Engineering,
YachayTech, Urcuquí. Ecuador.
