2024..01.01.25
Files > Conference Series > 2024 > Chimboazo ild pagina nueva
Proposal of a topical alternative for Cutaneous Leishmaniasis
Lilian Sosa *1,2, María Rincón 3,4
1 Instituto de Investigaciones en Microbiología (IIM), Universidad Nacional Autónoma de Honduras (UNAH), Tegucigalpa, Honduras, 11101 (LS).
2 Instituto de Investigaciones de Ciencias Aplicadas y Tecnológicas (IICAT), Universidad Nacional Autónoma de Honduras (UNAH), Tegucigalpa, Honduras, 11101 (LS).
3 Institut de Nanociència i Nanotecnologia (IN2UB), Universitat de Barcelona (UB), 08028 Barcelona, Spain (MR).
4 Departament de Ciència de Materials i Química Física, Facultat de Química, Universitat de Barcelona (UB), 08028 Barcelona, Spain (MR).
* Correspondence: [email protected]
Available from. http://dx.doi.org/10.21931/BJ/2024.01.01.25
Mr. Editor
Leishmaniasis is a group of diseases caused by a flagellated protozoan belonging to different species of the genus Leishmania, causing infections of the skin (cutaneous Leishmaniasis), mucous membranes (mucocutaneous Leishmaniasis) and internal organs (visceral Leishmaniasis)1. This disease is present in 88 countries worldwide, mainly in South and Central America, Africa, Asia and Southern Europe 2. In Honduras, this infection is endemic3, and by 2022, 1,565 new cases of cutaneous Leishmaniasis were reported4. The treatment of choice for all forms of this disease has been meglumine antimoniate, known commercially as Glucantime® (AMG), which is distributed for intravenous (IV) administration and produces adverse effects such as fever, nausea, vomiting, abdominal pain and nephrotoxicity. In some cases, it may be necessary to adjust the dosage of the drug or discontinue treatment if side effects are severe5. Another therapeutic option for the treatment of cutaneous Leishmaniasis is Amphotericin B deoxycholate (AMB), which, like AMG, is administered by IV but produces immediate adverse effects such as fever, chills, nausea, vomiting, headache, anaphylactic shock, arrhythmias and liver failure6. Although lipid formulations of AMB have been developed to reduce the toxic effects of the molecule and improve its effectiveness (liposomal, lipid complex, colloidal suspension), these presentations are expensive and make it impossible for patients to purchase this treatment. Likewise, there are less risky alternatives such as intralesional application with AMG and thermotherapy7. However, despite the efforts to research and develop new treatments, no topical treatment for cutaneous Leishmaniasis has been marketed. Topical treatments offer several advantages, including localized action, reduced systemic side effects, convenience of use and rapid absorption, making them a practical option for treating various medical and dermatological conditions. In this regard, we propose the development of a potential product that can be easily and rapidly prepared in a magistral formulation as a therapeutic alternative to Cutaneous Leishmaniasis.
Magistral formulations are personalized preparations of drugs by a pharmacist, specifically adapted to the individual needs of a patient under a physician's prescription, presenting advantages such as therapeutic innovation, cost reduction and access to specific treatments8. To this end, we pre-formulated a gel in which the AMG from a vial of Glucantime® was incorporated using a simple and low-cost vehicle such as Carbopol 940. To this end, we investigated the molecule's solubility in water, propylene glycol and glycerin, which are inexpensive and biocompatible vehicles.
Subsequently, a test to measure the biomechanical properties of the skin (transepithelial water loss) or TEWL (Transepidermal Water Loss) was performed as a preliminary test to determine whether humans could tolerate the formulation.
For the elaboration of the pre-formulation, 0.9 g of carbomer 940 was dispersed in water under gentle and constant stirring until a homogeneous translucent product was obtained with a pH ranging from 2.5 to 3.5. The gelation was completed by adjusting the pH by adding a sufficient amount of triethanolamine (trolamine) to a value of 7 (solution A). Then, an ampoule of Glucantime® and 0.5 g of gentamicin were dissolved in 10 ml of distilled water until completely dissolved (solution B). Subsequently, 59.5 g of solution B was added to solution A under constant stirring for 10 minutes. A very faint yellow creamy gel was obtained (Figure 1a).
To determine whether the gel could be tolerated on the skin, TEWL was measured with a Tewater® probe in 10 healthy female volunteers who were asked to sign an informed consent form and after approval by an ethics committee (reference number: IRB00003099). Participants were asked not to apply cream to the forearm area and were in a temperature-controlled room (20 ± 0.5 ◦C). The TEWL value was measured before, during and 4 hours after application of the AMG gel (Figure 1b). The average TEWL value at basal state was 10.26 ± 0.52 gm h-2-1. Subsequently, when the gel was applied, these values rose to 20.02 ± 2.79 gm h-2-1 due to the humidity caused by the gel itself; however, after 4 hours, the values decreased to 10.10 ± 0.73 gm h-2-1, a value similar to the basal state, indicating that there is no transepithelial water loss or dehydration once the gel is absorbed through the skin (Figure 2).

A B
Figure 1. A. AMG gel and B. Measurement of TEWL values in the forearm of one of the participants.
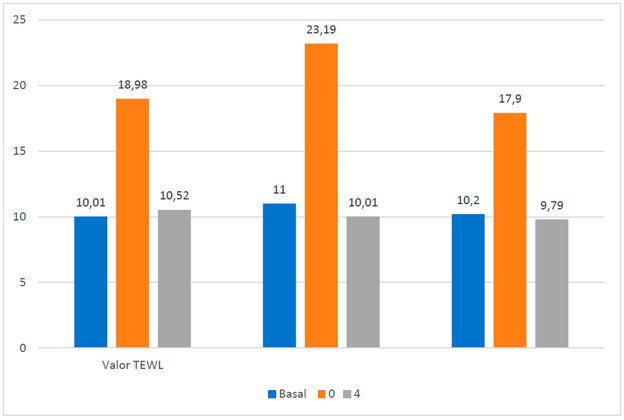
Figure 2. TEWL values. In blue, the basal values are without the AMG gel; in orange, the values are at the moment of placing the AMG gel on the skin; in gray, the values are after 4 hours of application of the AMG gel.
None of the participants exhibited itching, burning or discomfort in the area where the gel was applied. Also, the gel with AMG presented a pH value =5.6, indicating that it applies to the skin without damaging the skin barrier since the values belong to a eudermal pH9.
Finally, we concluded that this formulation was stable for one year without phase separation or color change, and further studies are required to evaluate the in vitro efficacy in different species of Leishmania as well as toxicity tests of this formulation in keratinocyte-like cell cultures (HaCaT). We believe AMG could be obtained in powder form to reduce manufacturing costs. It is important to note that not all cases of cutaneous Leishmaniasis can be effectively treated with topical treatments. However, the treatment choice will depend on the Leishmaniasis type, the lesions' extent and severity, and the patient's response to treatment. In some cases, combining topical treatments with systemic therapies may be necessary to achieve a complete cure for the disease, and it is essential to formulate topical preparations (adjuvant treatment)10. Treatment must be supervised by a physician specializing in infectious diseases or dermatology, who will evaluate the specific case and determine the most appropriate treatment plan for the patient.
Authors' contribution: LS and MR have conceptualized the idea. LS and MR have carried out the experiments. LS has performed the TEWL data analysis. LS and MR have written and prepared the paper. LS and MR reviewed and documented.
Conflicts of Interest: "We, the authors, declare that we have no conflicts of interest".
REFERENCES
1. Leishmaniasis. Available at: https://www.paho.org/es/temas/leishmaniasis (accessed February 01, 2024).
2. Pigott DM, Bhatt S, Golding N, Duda KA, Battle KE, Brady OJ, Messina JP, Balard Y, Bastien P, Pratlong F, Brownstein JS, Freifeld CC, Mekaru SR, Gething PW, George DB, Myers MF, Reithinger R, Hay SI. Global distribution maps of the leishmaniases. Elife. 2014 Jun 27;3:e02851. doi: 10.7554/eLife.02851.
3. Valladares, W, Escober, P, López, K, & Deras, A. Knowledge, attitudes and practices about Leishmaniasis in the departments of Cortés and Colón, Honduras. Revista De La Universidad, 2022; 1(2), 65-71. https://doi.org/10.5377/ru.v2i2.14584.
4. Honduras, Cutaneous and mucosal leishmaniasis 2022. Available at: https://panaftosa.org/leish/inf2023_es/INFOGRAFICO_HON_ESP_2023.pdf (accessed December 17, 2023).
5. Esfandiarpour I, Farajzadeh S, Rahnama Z, Fathabadi EA, Heshmatkhah A. Adverse effects of intralesional meglumine antimoniate and its influence on clinical laboratory parameters in the treatment of cutaneous Leishmaniasis. Int J Dermatol. 2012;51(10):1221-5. doi: 10.1111/j.1365-4632.2012.05460.x.
6. Drew, R.H.; Perfect, JR Conventional Antifungals for Invasive Infections Delivered by Unconventional Methods; Aerosols, Irrigants, Directed Injections and Impregnated Cement. J. Fungi 2022, 8, 212. https://doi.org/10.3390/jof8020212.
7. Frézard, F.; Aguiar, M.M.G.; Ferreira, L.A.M.; Ramos, G.S.; Santos, T.T.; Borges, G.S.M.; Vallejos, V.M.R.; De Morais, H.L.O. Liposomal Amphotericin B for Treatment of Leishmaniasis: From the Identification of Critical Physicochemical Attributes to the Design of Effective Topical and Oral Formulations. Pharmaceutics, 2023, 15, 99. https://doi.org/10.3390/pharmaceutics15010099.
8. Dumitriu Buzia, O.; Păduraru, A.M.; Stefan, C.S.; Dinu, M.; Cocoș, D.I.; Nwabudike, L.C.; Tatu, A.L. Strategies for Improving Transdermal Administration: New Approaches to Controlled Drug Release. Pharmaceutics, 2023, 15, 1183. https://doi.org/10.3390/pharmaceutics15041183.
9. Sensitive Skin Lab. Available at: https://sensilis.com/como-formulamos/ (accessed December 10, 2023).
10. Shmueli, M.; Ben-Shimol, S. Review of Leishmaniasis Treatment: Can We See the Forest through the Trees? Pharmacy, 2024, 12, 30. https://doi.org/10.3390/pharmacy12010030.
Received: October 9th 2023/ Accepted: January 15th 2024 / Published:15 February 2024
Citation: Sosa, L. and Rincon, M. Proposal of a topical alternative for Cutaneous Leishmaniasis. Bionatura Journal 2024; 1 (1) 25. http://dx.doi.org/10.21931/BJ/2024.01.01.25
Additional information Correspondence should be addressed to [email protected]
Peer review information. Bionatura thanks anonymous reviewer(s) for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
All articles published by Bionatura Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Publisher's Note: Bionatura Journal stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2024 by the authors. They were submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
