2024..01.01.37
Files > Conference Series > 2024 > Chimboazo ild pagina nueva
Epidemic survey of Crimean-Congo hemorrhagic fever infections in the Iraqi population during 2022
Riyadh A. Al-hilfi1, Raghad I. Kaleel1, Jinan J. Ghazzi2*, Hula Y. Fadhil2, Iman M. Aufi1, Ihab R. Aakef3, Hawraa A. Shakir1, Ahmed A. Hussain1, Zainab A. Mohsin1, Noora A. Abdulhadi1
1National Central Public Health Laboratory CPHL, Ministry of Health, Baghdad, Iraq.
2Department of Biology, College of Science, University of Baghdad, Baghdad, Iraq.
3Center for Disease Control, CDC, Ministry of Health, Baghdad, Iraq.
* Corresponding author: [email protected]
Available from. http://dx.doi.org/10.21931/BJ/2024.01.01.37
ABSTRACT
The primary cause of the Crimean-Congo hemorrhagic fever (CCHF) is a virus spread by ticks. The rate of frequency of case mortality is 10–40%, which is spurred on by the CCHF virus, which also produces severe viral hemorrhagic fever outbreaks. The current study can be viewed as an epidemiological survey of CCHF infections in Iraq, with the goal of better understanding block virus transmission and preventing the risk of contracting the disease. Approximately 1370 blood samples from suspect patients were taken and separated; then, the serum was processed to remove viral RNA and tested for CCHF infection using specialized kits. Results revealed 382 (27.9%) positive cases, including 76 (19.9%) positive patients with dying compared to 306 (80.1%) who experienced cures. Findings showed a significant incidence of more than 50% of the positive cases having contact with animals and raw meat, 33% slaughtering, and 36% tick bites.
Furthermore, 125 (32.7%) homemakers and 64 (16.8%) butchers had the most significant infection percentage. With 42.4% of all infections, Thiqar province had the most infected patients, followed by Misan with 9.7%, then Wasit and Babil. With 27.2% of all infections, May had the most CCHF-positive infections, followed by June and July. In conclusion, in response to the initial wave counterattack in southern Iraq, public health interventions on the veterinarian side should be implemented; these should eliminate, or at least decrease, the impact of a second wave. Illegal trading practices must be controlled if zoonotic diseases like CCHF are to stop spreading.
Keywords: CCHF, Iraqi provinces, RT-PCR, tick bites, zoonotic disease.
INTRODUCTION
A viral sickness identified as Crimean-Congo hemorrhagic fever (CCHF) is transmitted to humans by tick bites and direct interaction with contaminated human or animal blood or tissues. The primary tick vector's range encompasses Asia, Africa, the Balkans, and the Middle East, where CCHF was endemic. In Iraq, raising sheep and cattle is reasonably prevalent. Research has found that these animals frequently suffer from tick infestations, primarily with Hylomma species. An RNA virus from the Bunyaviridae family of the arbovirus genus Nairovirus causes CCHF, a devastating anthropogenic disease. The virus has encapsulated negative-stranded RNA. Depending on the manner of transmission and viral load, the incubation period for CCHF ranges from two to fourteen days (2–7 days following a tick bite or 10–14 days after blood transfusion). The clinical symptoms include preclinical sickness, acute infection with hemorrhage (petechiae, ecchymoses, epistaxis, pulmonary hemorrhage, intra-abdominal bleeding, haematuria, melena), and multi-organ failure1. Despite being named after its initial outbreaks in the Congo (1969) and Crimea (1944), it is no longer exclusive to these nations. CCHF is currently endemic to countries in Africa, the Caucasus, the Middle East, and a sizeable chunk of Asia, according to the World Health Organization (WHO)2. The global prevalence of acute CCHFV infections in humans is estimated to be 22.5%. The case mortality rate could range from 10% to 40%3.
Since the first incidence of CCHF was documented in 1979, Particularly in Iraq, the disease has already become endemic. The Iraqi health officials report several CCHF infections each year, but in the first half of 2022, warnings were raised due to the disease's rising burden. Tick infestations in livestock animals frequently put linked individuals in danger. One of the causes of the outbreak is thought to be the COVID-19-related inadequacy of livestock spraying over the previous two years. That is highly concerning in Iraq, where grazing cattle is popular. The objective of the current study is to gain a better knowledge of how to stop viral transmission and lower the risk of contracting the disease. It can be seen as an epidemiological survey of CCHF infection in Iraq.
MATERIALS AND METHODS
Data source
One thousand three hundred seventy suspected blood specimens were collected from January to December 2022 in 18 Iraqi provinces. Serum was extracted from specimens and transported, cooled in ice, to the central public health laboratory (CPHL) in Baghdad for diagnosis. Specimens were collected from patients aged 1–99 years old (790 males and 580 females) with different social levels (student, Private work, Retired, Housewife, Butcher, Employee, Driver, Animal owner, livestock grazing, Farmer, Market worker and others). They were in direct contact with Confirmed cases, raw meat and animals, according to Table 1.
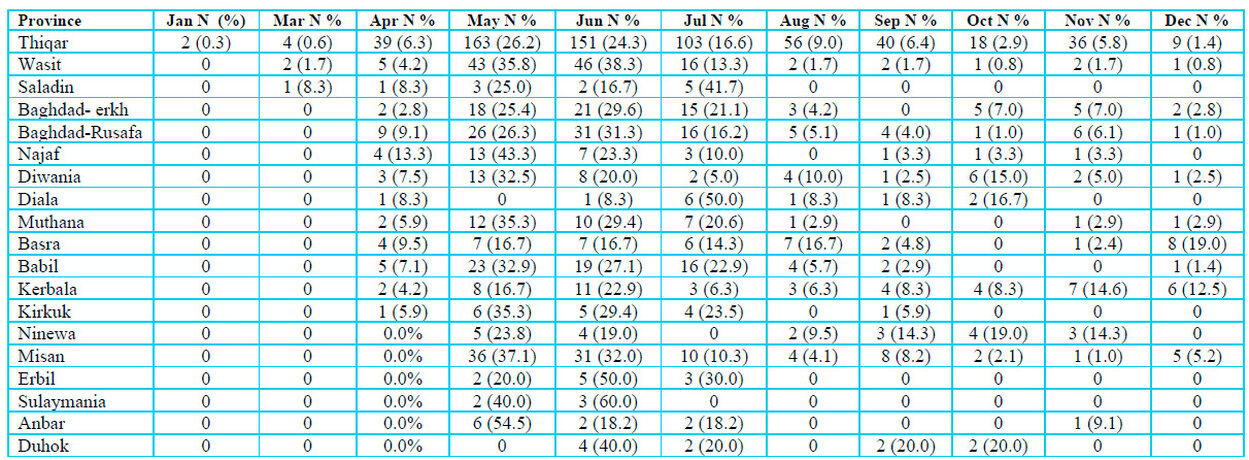
Table 1: Distribution of Crimean-Congo hemorrhagic fever suspected specimens stratified to Iraqi provinces during months in 2022.
Viral nucleic acid extraction
Using a specialized laboratory kit (QIAamp viral RNA mini kit, QIAGEN, Germany), viral RNA was isolated from the specimens obtained according to the manufacturer's instructions. Prior to CCHF testing using real-time reverse transcriptase polymerase chain reaction (rRT-PCR) or ELISA procedures, the viral RNA was kept at -70 °C.
Diagnosis of CCHF infection
Real-Time Reverse Transcriptase PCR Analysis
Real-time reverse transcriptase PCR analysis was performed on the produced RNA using a specific detection kit (The RealStar® CCHFV RT-PCR Kit 1.0, Altona Diagnostics GmbH, Germany). It is an in-vitro diagnostic test for detecting Crimean-Congo hemorrhagic fever (CCHF) virus-specific RNA based on real-time PCR technology. The real-time PCR thermal cycler was used with the plate (QuantStudioTM) with the following amplification steps: reverse transcription holding step at 50 °C for 10 minutes, denaturation holding phase at 95 °C for 2 minutes, then 45 cycles of amplification at 95, 55, and 72 °C, respectively. The fluorescence detectors dye CCHFV specific RNA CCHFV FAM TM showed a positive result.
Serological analysis by using the ELISA technique
CCHF-IgM Human Crimean-Congo Hemorrhagic Fever Virus ELISA Kit (Abbexa LTD, Cambridge, UK) was used to determine levels of CCHFV antibody in the serum of suspected patients (with disease outcome and negative PCR results). This kit is based on enzyme-linked immune-sorbent assay technology.
Statistical evaluation
GraphPad Prism 8.0.0 and IBM SPSS Statistics 25.0 (IBM Corp., Armonk, NY) are statistical software programs utilized to manage and carry out this analysis of the frequencies, percentages, and graphs. When data for continuous variables are not normally distributed, the normality test is used to characterize the median with an interquartile range, and the Kruskal-Wallis test is used to compare continuous variables. The two-tailed Fisher exact test or the Pearson Chi-square test compares categorical variables, reported as numbers and percentages. A probability (p) value of 0.05 was used to define statistical significance.
RESULTS
Baseline characteristics of CCHF patients
A total of 1370 suspected blood samples were taken throughout 2022, and they were divided into positive 382 (27.88⸓) and negative 988 (72.11⸓), as described in Table 2. Patient outcomes showed a highly significant correlation between positive and negative groups (p˂0.001), with the death of 76 (19.9⸓) positively diagnosed patients and 38 (3.8⸓) negative cases. Meanwhile, cured cases were 306 (80.1⸓) of the positive group. Results also revealed that 137 (36⸓) of the positive cases and 157 (15.9⸓) of the negative cases were subjected to tick bites (P˂0.001). The presence of rodents in 170 (44.5⸓) of the positive patients and 274 (27.7⸓) in the opposing group, which means that the presence of rodents also had a highly significant correlation with infection (P˂0.001). Additionally, this study's results demonstrate that 207 (54.3⸓) of the infected patients and 273 (27.7⸓) of the negative cases had contact with animals, and 210 (55⸓), 184 (18.6⸓) had contact with raw meat, while 127 (33.3⸓) of the positive cases and 95 (9.6⸓) of the negative cases were butchers. Cope with case definition had a highly significant correlation (P˂0.001) between positive 282 (74⸓) and negative 460 (46.6⸓) cases.
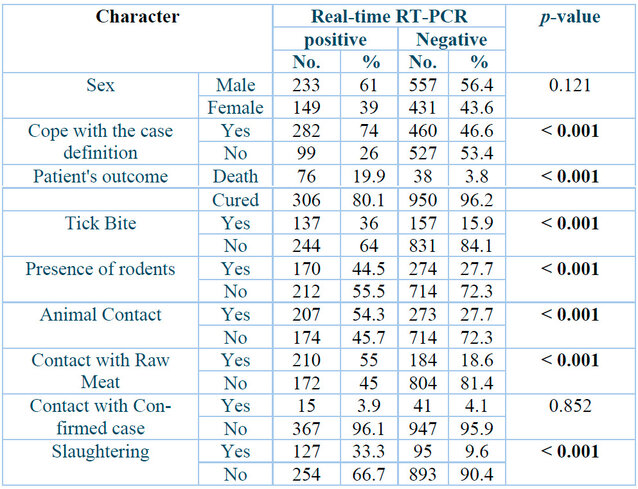
p: Probability of Two-tailed Fisher exact test (significant p-value is indicated in bold).
Table 2: Baseline characters of Crimean-Congo hemorrhagic fever patients.
Manifestations of CCHF patients
Table 3 shows the most common clinical signs between the positive and negative groups. Firstly, bleeding at injection sites had the highest significant correlation between the two study groups (P˂0.001), where 111(29.1⸓) individuals and 135 (13.7⸓) of the negative individuals suffered from this type of bleeding, followed by bleeding of mouth 71(18.4⸓) positive and 98 (9.9⸓) of the negative individuals suffer from mouth bleeding (P˂0.001). Secondly, fever and ecchymosis both had a highly significant correlation with the two study groups (P= 0.001), where 361 (94.5⸓) positively diagnosed patients and 878 (88.9⸓) negative people suffered from high fever and 105 (27.6⸓) and 190 (19.2⸓) people showed signs of ecchymosis.
Table 3 also shows a statistically significant relationship between the two study groups and bleeding of GTI (p= 0.036), where 17 (4.5⸓) positive patients and 23 (2.3⸓) negative people exhibit this bleeding type.
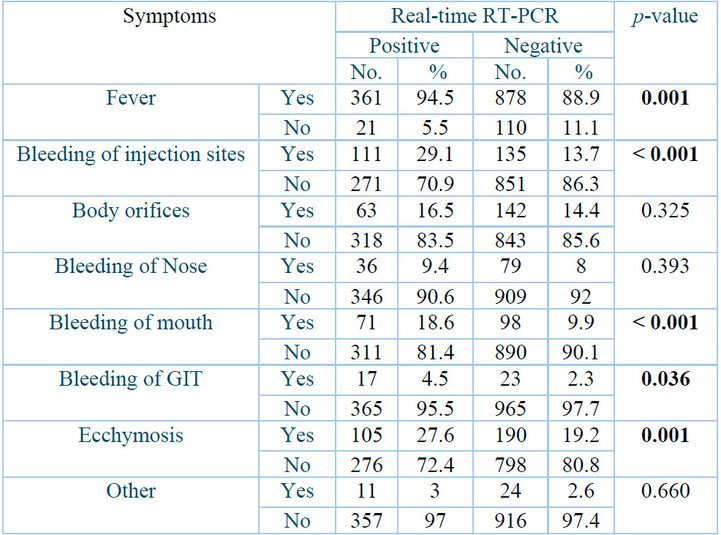
p: Probability of Two-tailed Fisher exact test (significant p-value is indicated in bold).
Table 3: Clinical signs of Crimean-Congo hemorrhagic fever patients.
CCHF infections stratified to the patient's occupation
Table 4 demonstrates that the highest infection percentage, 125 (32.7⸓) of infected individuals were homemakers, and 64 (16.8⸓ ) were butchers. People with other occupation types had 83 (21.7⸓) infections, while students had 35 (9.2⸓) infections.
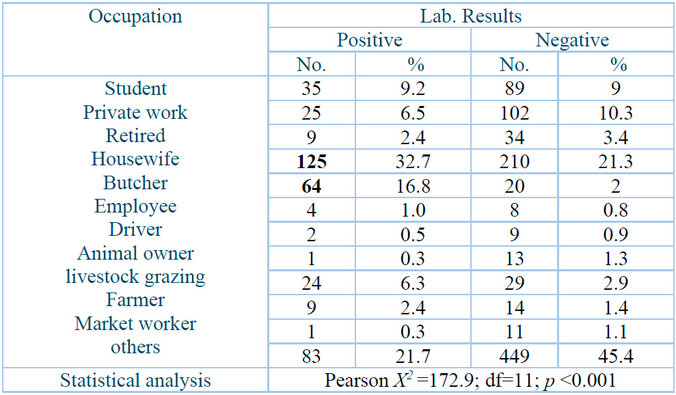
D.F.: Degree of freedom; p: Probability.
Table 4: Crimean-Congo hemorrhagic fever infections stratified to patient occupation.
Distribution of CCHF-infections in Iraqi provinces
Figure 1 demonstrates the distribution of positive CCHF-infected cases throughout 18 Iraqi provinces. This province had the highest number of infected patients, with 42.4 ⸓ of total infections, followed by Misan at 9.7⸓ then Wasit and Babil with 6.8⸓ and 6.3 ⸓ respectively. The current study also showed that Muthana and Basra provinces had almost the same percentage of infections, which were 5.2⸓ and 5⸓, respectively. Kerbala and Diwania provinces both had 4.5⸓ of total infections. Baghdad province was divided into Kerkh, 3.9 ⸓ infections, and Rusafa, 3.4⸓ infections. Ninewa in the north of Iraq had 2.4⸓ infections; meanwhile, Anbar province in the west of Iraq had the lowest percentage of infections, only 0.3 ⸓.
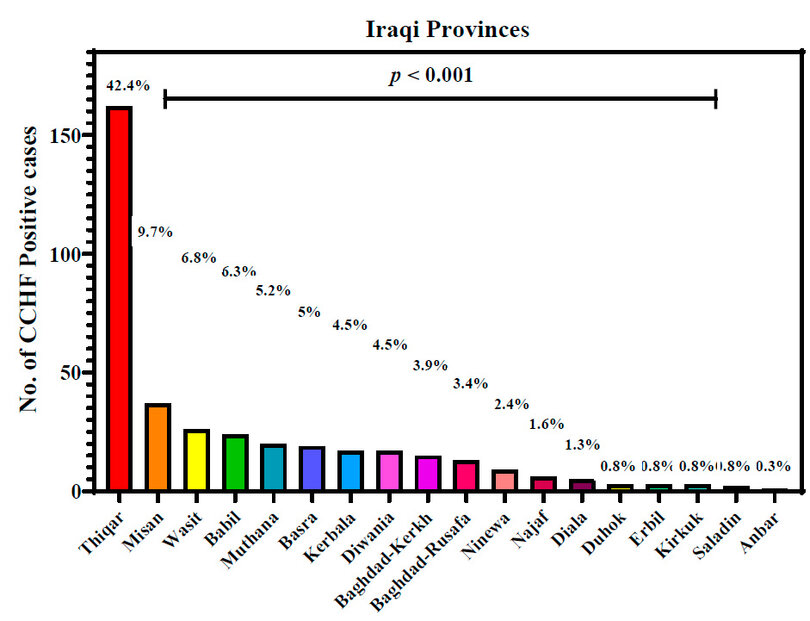
Figure 1: Crimean-Congo hemorrhagic fever infections in Iraqi provinces during 2022. p: probability of Pearson Chi-square test (to compare categorical variables).
Appearance of CCHF infections in the Iraqi population during the months of 2022
Figure 2 explains the appearance of CCHF infections in the Iraqi population distributed during different months of 2022. May had the highest number of CCHF-positive infections with 27.2⸓ of total infections, followed by June of 24.3⸓ then July of 14.4⸓ positive infections. April and August had almost similar infections of 7.3⸓ and 7.6⸓ infection percentage, respectively. Infected ceases to appear to decrease in number and percentage during cooler months of the year were 5.5⸓ in September, 3.9⸓ during October, 6⸓ in November and 3.1⸓ in December. May had the lowest infection percentage of 0.5.
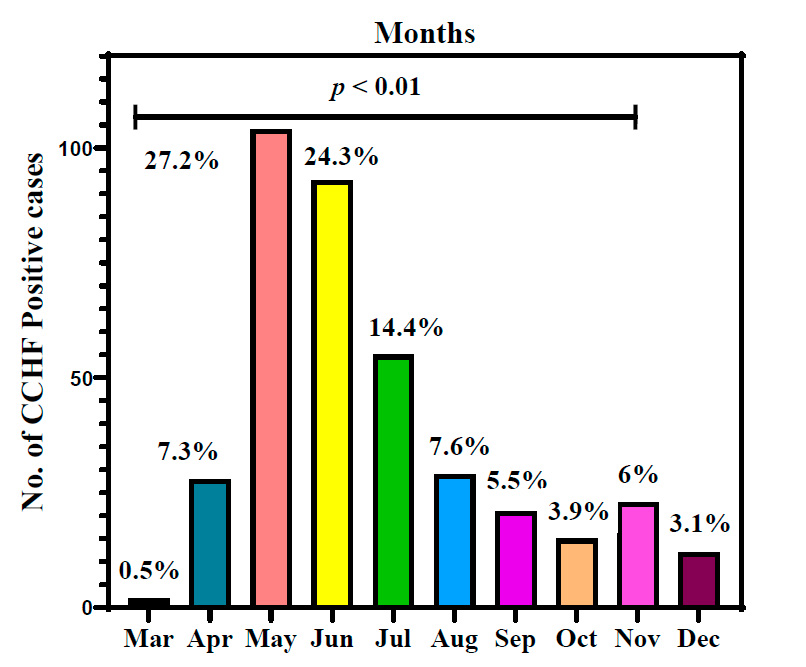
Figure 2: Appearance of Crimean-Congo hemorrhagic fever infections in the Iraqi population during 2022. p: probability of Pearson Chi-square test (to compare categorical variables).
Correlation of age with the median of positive cases
This research categorized 314 confirmed positive cases into five age demographics. The first age group of ≤15 years old had 28 (7.3⸓) positive patients, the second group of 16-30 years had the highest number of infected persons, 127 (33.2⸓), and the third group of 31-45 years had 123 (32.2⸓) patients. People aged 46-60 years had 75 (19.6 ⸓) infections, and older people aged 61 - ≥75 years had the lowest infection percentage of 29 (7.6⸓).
Figure 3 shows that age groups are highly related to age median (in the interquartile range) during CCHF infections (P˂0.01). Results showed that the 16-30 and 31-45 age groups had higher infection percentages, infection frequencies, and higher age medians. In contrast, the 61-≥ 75 and ≤ 15 age groups had lower infection frequencies and lower age median. This result proved that age has a strong relation to infection with CCHF.
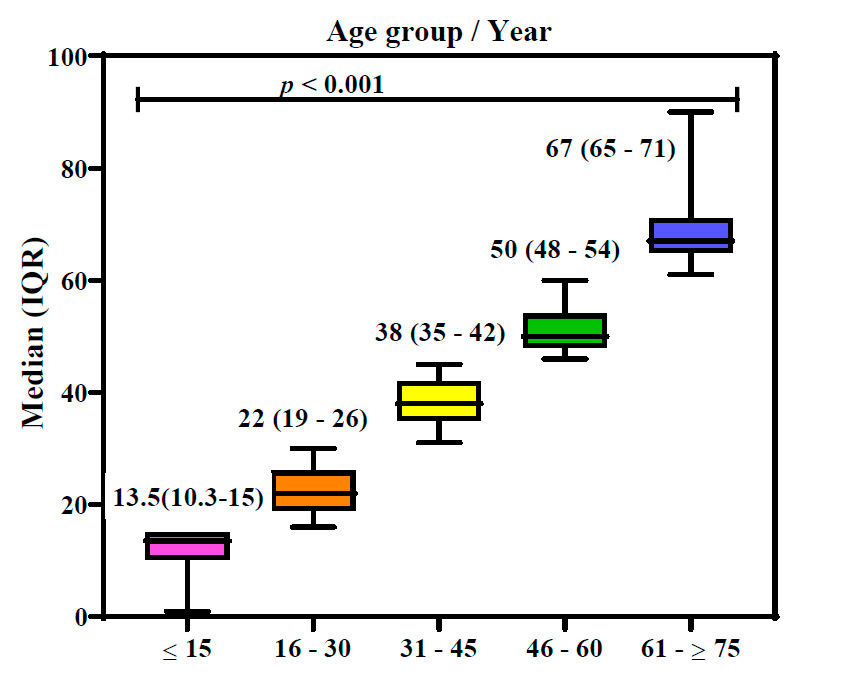
Figure 3: Box plot of ages in Crimean-Congo hemorrhagic fever patients. p: probability of Kruskal-Wallis test (to compare continuous variables).
DISCUSSION
A total of 1370 suspected specimens were incorporated in this study, with 382 positively confirmed cases, 114 deaths, and 1256 cured cases. Due to the elevated number of dead cases of patients involved in this study, it is crucial to understand the rote of transmission of CCHFV to humans. The results of this investigation were in agreement with other studies, which indicated that domestic cattle serve as the disease's main hosts and can spread it to people while it is still in the viremic stage. High-risk behaviors, including unauthorized animal slaughter, handling and/or inspecting livestock without gloves or protective masks, and slaughtering animals, make the disease more contagious. Also, health professionals risk acquiring an infection from patients' blood and bodily fluids6. The serological incidence of CCHFV in patients with a history of tick bites may approach 20% in endemic areas7. Living in a rural neighborhood is a risk factor for exposure to the tick vector and acquiring CCHFV.
Nevertheless, because of the high number of animal markets close to major cities, CCHFV serological frequency is also higher in urban than rural residents5. It suggests those two groups had a history of working with animals, handling contaminated animal carcasses, blood, or red meat without donning protective clothing such as gloves and masks, wearing work clothing and boots, or interacting with animals. It is conceivable that they were acquainted with ticks while holding the knife in their mouth, dressing animals, handling animal blood, or interacting with an animal carcass in the past. High-risk behaviors associated with the workplace have been proven to increase the risk of infections more than individual awareness and performance do11.12. Butchers who work at slaughterhouses frequently indulge in dangerous activities like eating raw liver, dressing animals while holding the knife in their lips, and dressing animals while wearing the incorrect footwear and attire13. Surveys in the Iranian provinces of Ilam and Mazandaran found that 75.3% and 73.8% of respondents did not use protective covers14,15 when handling livestock or cleaning their platform.
Moreover, studies have revealed that 5.9% of CCHF-positive individuals in Qom Province and 38% of CCHF-positive cases in Khorasan Province mentioned consuming raw liver or coming into contact with animal blood16. With the correct data, these people can change their patterns of behavior. For instance, a Turkish study shows that a person's literacy level directly impacts whether they utilize protective covers17. These folks can alter their habits and behavior with the correct information. For instance, a Turkish study demonstrates that a person's reading level directly affects whether they use protective covers18. Results also reveal that gender had an indirect relation with infection.
As observed in this study and elsewhere9, Pathological changes among patients with CCHF varied significantly, from vague symptoms to severe multi-organ failure and fulminant bleeding. Fever, bleeding from the nose, mouth, GTI, and injection sites are frequent in our study, and ecchymosis was frequently observed in the patients. Furthermore, despite the sparse data, thrombocytopenia was found in every case, and other papers state that this symptom is a typical hemo-pathological aberration in CCHF patients10.
The current investigation results revealed that the prevalence of infection was higher in May, June, and July when summertime temperatures are higher, and ticks are more likely to be present. Meanwhile, during the colder months, infection rates were lower. The rise in challenging tick infestations of livestock and farms in 2022 can be used to explain the rise in CCHF cases in Iraq. The lack of pest management efforts during the coronavirus disease 2019 (COVID-19) pandemic in 2020 and 2021 may be responsible for this increase. Additionally, farmers, butchers, and general public health workers must be made aware of CCHF and its method of transmission. This significantly contributes to the rise in sickness rates since people are more susceptible to contracting the virus if they have a history of tick bites or animal contact. Most CCHF cases occur in rural areas where wild and domestic animals serve as tick hosts, resulting in viremia that helps maintain the virus and spread throughout the ecosystem19. The only complex tick species recognized as a CCHF vector in Iraq is Hylomma marginatum. The data suggest that 28 other species from seven additional genera, including Hyalomma, Rhipicephalus, Boophilus, Ambylomma, Haemaphysalis, and Ixodes, may be capable of disseminating the virus 20.
Meanwhile, studies have yet to report on discovering these species in Iraq. The most frequent tick discovered in Ghanaian cattle is the three-host A. variegatum, one of the most hazardous ticks in Africa 20. It must be anticipated that CCHFV-positive pools in ticks derived from cattle pose a risk to human health because viruses can propagate through other hosts, such as wild animals and birds.
The outcomes of this research also suggest that although trade in live animals, meat, and meat products can act as a mobile pool of diseases like CCHF, possibly having significant adverse effects on both the economy and public health, even though meat and meat products are an essential source of nutrition 21.
Also, multiple studies have demonstrated the influence of the climate on tick activity and CCHF incidence. Further research examining the relationship between environmental circumstances and the occurrence of CCHF could be beneficial for developing early warning systems to monitor this disease 22.
The current study revealed that younger patients at the age ≤ 15 years may be infected from tick bites or contact with infected patients, and they were lower in number than the 16 -30 and 31-45 age groups that had a higher number of infections due to their occupation as butchers, homemakers, or animal grazing. Older individuals had lower infections because they were too old to work.
CONCLUSIONS
This article discusses the epidemiology of the 2022 CCHF outbreak in Iraq, which started in January 2022. The Lancet's July 2022 infectious diseases monitoring report noted an upsurge in instances. The median age of the confirmed cases, primarily male and located in southern Iraq, was 22 years (IQR: 19–26). For patients, the mortality rate was 19.9%. The outbreak is currently expected to last until June 2022, which demonstrates the CCHF's seasonality that was previously noted. In March 2022, the first confirmed case was found. A second wave appeared in August through October 2022, according to research on the seasonality of CCHF. Comprehensive public health interventions on the veterinary side should be implemented in response to the first wave in southern Iraq; these should prevent or minimize the severity of a second wave. Transnational animal trafficking is probably the reason why the majority of cases were concentrated in the Iranian border governorates in southern Iraq. Illegal trading practices must be controlled if zoonotic diseases like CCHF are stopped from spreading.
Financial support and sponsorship: This research received no external funding
Conflict of interest: The authors declared no conflict of interest.
Sources of funding: This research did not receive any specific grant from public, commercial, or not-for-profit funding agencies.
REFERENCES
1. Shayan S, Bokaean M, Shahrivar MR, Chinikar S. Crimean-Congo hemorrhagic fever. Lab Med. 2015;46(3):180–189. DOI: 10.1309/LMN1P2FRZ7BKZSCO.
2. CDC, Crimean-Congo Hemorrhagic Fever (CCHF). Centers for Disease Control and Prevention; 2022. https://www.cdc.gov/vhf/crimean-congo/index.html.
3. CDC, Crimean-Congo Hemorrhagic Fever (CCHF). Centers for Disease Control and Prevention; 2022. https://www.cdc.gov/vhf/crimean-congo/prevention/.
4. World Health Organization, WHO Coronavirus Disease (COVID-19) Dashboard with Vaccination Data; 2021. https://www.tandfonline.com/doi/full/10.1080/08830185.2020.1800688.
5. Chinikar S, Goya MM, Shirzadi MR, et al. Surveillance and laboratory detection system of Crimean-Congo hemorrhagic fever in Iran. Transbound Emerg Dis. 2008;55(5–6):200–204. DOI: 10.1111/j.1865-1682.2008.01028.x.
6. Celikbas AK, Dokuzoğuz B, Baykam N, et al. Crimean-Congo hemorrhagic fever among health care workers, Turkey. Emerg Infect Dis. 2014;20(3):477–479. DOI: 10.3201/eid2003.131353.
7. Uyar Y, Christova I, Papa A . Current Crimean Congo hemorrhagic fever (CCHF) situation in Anatolia and Balkan Peninsula. Türk Hij Den Biyol Derg. 2011;68(3):139–151. DOI: 10.5505/TurkHijyen.2011.60352.
8. Izadi S, Holakouie-Naieni K, Majdzadeh S, et al. Seroprevalence of Crimean-Congo hemorrhagic fever in Sistan-va-Baluchestan province of Iran. Jpn J Infect Dis. 2006;59(5):326–328. DOI: 10.1016/j.ijid.2003.10.008.
9. Al-Abri SS, Hewson R, Al-Kindi H, et al. Clinical and molecular epidemiology of Crimean-Congo hemorrhagic fever in Oman. PLoS Negl Trop Dis. 2019;13(4):e0007100. Published 2019 Apr 25. DOI:10.1371/journal.pntd.0007100.
10. Spengler JR, Bente DA. Crimean-Congo Hemorrhagic Fever in Spain - New Arrival or Silent Resident?. N Engl J Med. 2017;377(2):106-108. DOI:10.1056/NEJMp1707436
11. Akuffo R, Brandful J, Zayed A, Adjei A, Watany N, Fahmy N, et al. Crimean-Congo hemorrhagic fever virus in livestock ticks and animal handler seroprevalence at an abattoir in Ghana. BMC Infect Dis. 2016;16(1):324–329. DOI: 10.1186/s12879-016-1660-6.
12. Atif M, Saqib A, Ikram R, Sarwar MR, Scahill S. The reasons why Pakistan might be at high risk of Crimean Congo hemorrhagic fever epidemic; a scoping review of the literature. Virol J. 2017;14(1):63–72. DOI: 10.1186/s12985-017-0726-4.
13. Chinikar S, Moghadam AH, Parizadeh SJ, et al. Seroepidemiology of crimean congo hemorrhagic Fever in slaughterhouse workers in northeastern Iran. Iran J Public Health. 2012;41(11):72-77.
14. Sharifinia N, Rafinejad J, Hanafi-Bojd A, Biglarian A, Chinikar S, Baniardalani M, et al. Knowledge and attitudes of the rural population and veterinary and health personnel concerning Crimean-Congo Hemorrhagic fever in western Iran in 2012. Fla Entomol. 2013;96(3):922–928. DOI: 10.1653/024.096.0328.
15. Ziapour SP, Kheiri S, Mohammadpour RA, Chinikar S, Asgarian F, Mostafavi E, et al. High-risk behavior and practice of livestock and meat industry employees regarding Crimean-Congo Hemorrhagic fever in Nur County, northern Iran. J Mazandaran Univ Med Sci. 2016;25(132):49–61.
16. Saghafipour A, Norouzi M, Sheikholeslami N, Mostafavi R. Epidemiologic status of the patients with Crimean Congo Hemorrhagic fever and its associated risk factors. J Mil Med. 2012;14(1):1–5.
17. Arıkan I, Kasifoglu N, Metintas S, Kalyoncu C. Knowledge, beliefs, and practices regarding tick bites in the Turkish population in a rural area of the middle Anatolian region. Trop Anim Health Prod. 2010;42(4):669–675. DOI: 10.1007/s11250-009-9474-9
18. Mostafavi E, Haghdoost A, Khakifirouz S, Chinikar S. Spatial analysis of Crimean Congo hemorrhagic fever in Iran. Am J Trop Med Hyg. 2013;89(6):1135–1141. DOI: 10.4269/ajtmh.12-0509.
19. Spengler JR, Bergeron É, Spiropoulou CF. Crimean-Congo hemorrhagic fever and expansion from endemic regions. Curr Opin Virol. 2019;34:70-78. DOI:10.1016/j.coviro.2018.12.002.
20. Akuffo R, Brandful JA, Zayed A, et al. Crimean-Congo hemorrhagic fever virus in livestock ticks and animal handler seroprevalence at an abattoir in Ghana. BMC Infect Dis. 2016;16:324. Published 2016 Jul 8. DOI:10.1186/s12879-016-1660-6.
21. Drosten C, et al. Rapid detection and quantification of RNA of Ebola and Marburg viruses, Lassa virus, Crimean-Congo hemorrhagic fever virus, Rift Valley fever virus, dengue virus, and yellow fever virus by real-time reverse transcription-PCR. J Clin Microbiol. 2002;40:2323–2330. DOI: 10.1128/JCM.40.7.2323-2330.2002.
22. Ansari H, et al. Crimean-Congo hemorrhagic fever and its relationship with climate factors in southeast Iran: a 13-year experience. J Infect Dev Ctries. 2014;8:749–757. DOI: 10.3855/jidc.4020.
Received: October 9th 2023/ Accepted: January 15th 2024 / Published:15 February 2024
Citation: Al-hilfi R. A., Kaleel R. I., Ghazzi J. J., Fadhil H. Y., Aufi I. M., Aakef I. R., Shakir H. A., Hussain A. A., Mohsin Z. A., Abdulhadi N. A. Epidemic survey of Crimean-Congo hemorrhagic fever infections in the Iraqi population during 2022. Bionatura Journal 2024; 1 (1) 37. http://dx.doi.org/10.21931/BJ/2024.01.01.37
Additional information Correspondence should be addressed to [email protected]
Peer review information. Bionatura Journal thanks anonymous reviewer(s) for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
All articles published by Bionatura Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Publisher's Note: Bionatura Journal stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2024 by the authors. They were submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
