2024..01.01.34
Files > Conference Series > 2024 > Chimboazo ild pagina nueva
Chronic Kidney Disease affects Thyroid Hormones
Tiba Mohammed Jadaan1, Haitham L. Al-Hayali2
1Department of Biology, College of Science, Mosul University, Mosul, Iraq; E-mail: [email protected]. ORCID 0009-0008-4933-7671
2Department of Biology, College of Science, Mosul University, Mosul, Iraq;
E-mail: [email protected]. ORCID 0000-0002-8937-9285
* Correspondence: [email protected].
Available from. http://dx.doi.org/10.21931/BJ/2024.01.01.34
ABSTRACT
Kidney disease is one of the causes of death in many countries around the world. This study found that chronic kidney disease affects thyroid hormone formation, release, and storage. The results show a decrease in T3 and T4 thyroid hormones and an increase in TSH hormone in both sexes and people of different ages. The research aims to study the effect of chronic kidney disease on the thyroid gland’s activity and its deficiency’s effect on health ailments. Results showed a significant decrease in T3 concentration in patients at the probability level (p ≤ 0.05) (0.8±0.1) compared with the control group (1.0±0.1).
Additionally, a significant decrease in T4 concentration at the probability level (p ≤ 0.05), (6.8±1.5) compared with the control group (8.7±0.9) and a significant increase in TSH concentration at the probability level (p ≤ 0.05), (4.8±0.6) compared with the control group (1.2±0.5). The CDK affects thyroid hormones; low T3 and T4 are the most common thyroid dysfunction. High TSH, enlarged thyroid gland, hypothyroidism, thyroid dysfunction.
Keywords: CKD, Thyroid Hormones, Thyroid dysfunction.
INTRODUCTION
It has been found that there is an increase in the prevalence of chronic kidney disease in all parts of the world it used to be. Today, the disease affects about 10-15% of the adult population with different age stages. Furthermore, the percentage of people over 60 years was higher than the rest of the ages, as the infection rate reached about 39%, compared to ages between 40-50 years, when the infection rate reached about 13%.
The kidney disease family history is a risk factor for chronic kidney disease. Some sources illustrate that 24% of patients who suffer from it in its final stages have at least a first-degree relative, as well as a previous history of urinary tract problems, urinary tract obstruction, stones, decrease in kidney mass (single kidney), nephrotoxins, polyuria analgesic abuse, low birth weight1.
Kidney damage is defined as a chronic disease that affects the kidneys and leads to a defect in its structure or function, causing kidney weakness, which ranges from mild damage to kidney failure, as the kidneys cannot get rid of excess nitrogenous waste, salts, and excess water from the body’s need. The disease begins without a decrease in the glomerular filtration rate (GFR), but a decrease occurs over time and delayed treatment2. CKD affects about 8-16% all over the world. Diabetes and high blood pressure are among the leading causes of the disease in developed countries. The rate of early detection of it is at most 5% 3.
The thyroid gland produces three hormones, which play a role in the development and functioning of the kidneys and the state of salt and water balance in the blood and the body. Low thyroid activity decreases the amount of water absorbed by the kidneys. Subclinical hypothyroidism is the most common disorder of the gland in CKD patients4.
The growing body of evidence shows that thyroid dysfunctions such as low circulating levels of triiodothyronine and hypothyroidism are related to a higher risk of CVD and death in hemodialysis patients.
MATERIALS AND METHODS
Study samples
The study included 54 males and females randomly ill cases, with ages ranging from 48 -76 years, who attended private kidney and urology clinics and private medical laboratories in the city of Mosul, and who were diagnosed with chronic kidney disease by specialized doctors for the period from September 2020 to June 2022. It included 30 males and 24 females. In addition, 30 healthy 15 males and 15 females were in the control group.
Collect Blood Samples
5.0 ml of venous blood was drawn after fasting for a period ranging between 10-12 hours, then blood samples were placed in a gel tube and left for 15 minutes until clot formation using centrifugation at 3000 rpm for 10 minutes to separate Serum while excluding decomposed specimens. The blood serum was divided into 1.5 ml Eppendorf tubes and then kept at a temperature of -80 °C using a deep freezer for later use.
Hormonal measurements
The hormones measured are the thyroid-stimulating hormone (TSH), the triiodothyronine (T3) and the tetra iodothyronine (T4), using the technique of the Automated Immunoassay Analyser (AIA-360) equipped by the Japanese company TOOSOH (The research is extracted from a master’s thesis and contains the part, not all the information, just hormones)
Statistical Analysis
The data were analyzed according to the system of simple and universal experiments using a completely randomized design. The different information was significantly distinguished by different alphabet letters under the probability level of 1% and performed by Duncan’s test at SPSS version 266.
RESULTS
The results of the effect of CKD on thyroid hormones are shown in Tables (1) and (2). There is a significant decrease in T4 and T3 compared with the control group; the differences were at different ages and for both sexes, directly proportional to the progression of chronic kidney disease. As the patient gets older, the impact of CKD becomes more severe and may lead to death. Conversely, there is a significant increase in TSH compared to the control group due to a deficiency of thyroid hormones. The differences were at different ages and for both sexes.
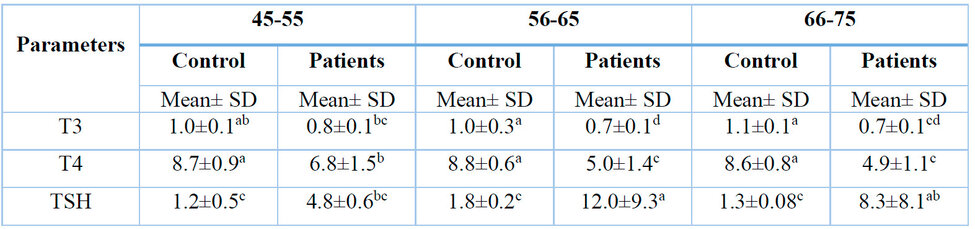
*According to the Duncan test, the different numbers horizontally indicate significant differences at the probability level p ≤ 0.05.
Table1: Effect of kidney disease on male petition at different ages.
The results showed a significant decrease in T3 concentration in patients at the probability level p ≤ 0.05 compared to the control group. Additionally, there was a significant decrease in T4 concentration at the probability level p ≤ 0.05 compared with the control group.
The results also showed a significant increase in TSH concentration at the probability level p ≤ 0.05 compared with the control group.
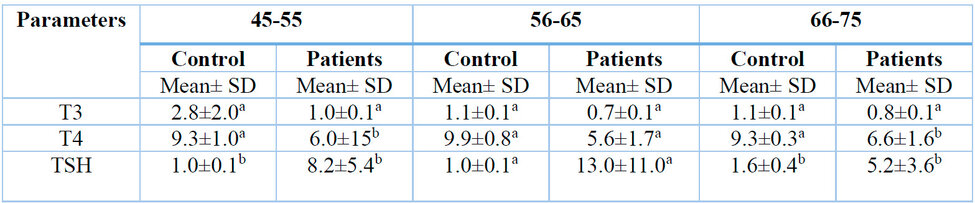
* According to the Duncan test, The different numbers horizontally indicate significant differences at the probability level p ≤ 0.05.
Table 2: Effect of kidney disease on female patients at different ages.
Table 2 (A physician diagnosed the samples taken as having chronic kidney disease and the effect only on hormones) illustrates the effect of CKD on thyroid hormones in females of different ages between 45 and 75. The results showed a significant decrease in T3 and T4 concentration compared with the control group. The results also indicate a significant increase in TSH concentration compared with the control group.
DISCUSSION
The result showed a high prevalence of thyroid dysfunction. These are consistent with that illustration7. Chronic kidney disease can alter thyroid hormone secretion, metabolism, synthesis and degradation, presenting different clinical syndromes of thyroid dysfunction. Multiple mechanisms could explain these syndromes: alteration of peripheral hormone metabolism, Decreased concentration of T.H., disturbed binding to carrier proteins, increased iodine stores in thyroid glands and possible reduction in tissue thyroid content8.
Triiodothyronine is the most metabolically active thyroid hormone, for instance, could be dropped in CKD patients even with an average TSH level. This is termed as ‘Low T3 Syndrome’. Thyroid dysfunction might appear as one of the subsequent: Thyroid enlargement, thyroid hormone deficiency or excess (hyperthyroidism or hypothyroidism); asymptomatic or symptomatic (the subclinical state or overt)9. The thyroid gland affects metabolic processes in the body, and the research supports a link between thyroid and kidney function. Patients with end-stage kidney disease and chronic kidney disease are at risk of developing hypothyroidism10.
Thyroid function can also affect kidney function, the development of chronic kidney disease, and increase the risk of cardiovascular disease. CKD patients have the highest risk of cardiovascular disease, and impaired thyroid function may lead to increased CVD risks as well as mortality, as shown for patients with ESKD11.
Reports indicate that CDK development is linked with complications such as hypothyroidism, dyslipidemia, and cardiovascular disease. Usually, the kidneys play an essential role in the degradation, metabolism and secretion of thyroid gland hormones. Chronic kidney disease affects the hypothalamic-pituitary-thyroid axis. Chronic kidney disease affects thyroid function in several ways, including altered iodine storage in the thyroid gland, altered metabolism of peripheral hormone, decreased tissue thyroid hormone content, insufficient binding to carrier proteins and decreased thyroid hormone levels. Thus, in chronic kidney disease, thyroid hormone metabolism is impaired, and chronic kidney disease is associated with primary hypothyroidism12.
On the other hand13, we reported that 10.6% of CDK cases in the community, in the presence of diabetes and advanced age, are one of the factors that predict the disease. We found a higher prevalence of thyroid gland dysfunction in CKD patients. A hemodialysis patients in western Nepal study indicates a prevalence of clinical and subclinical hypothyroidism of 26.6% of patients14.
Thyroid-stimulating hormone (TSH) is produced from the anterior pituitary gland and promotes the inhibition and stimulation of thyroid hormone secretion from the gland. TSH production is controlled by a hypothalamic hormone called thyrotropin, depending on developmental, environmental, and circadian stimuli15.
Chronic kidney disease affects the hypothalamic-pituitary-thyroid axis, the central control axis for thyroid hormones and metabolism. Primary hypothyroidism is common in
CKD patients who have a low estimated (GFR). Low T3 syndrome is the most common disorder in patients with CKD. T4 levels are also affected because of impaired protein binding of T416. Several factors are associated with T3 reduction in patients with CKD, such as systemic acidosis, markers of endothelial damage and inflammation. The 1,5′-deiodinase enzyme is responsible for converting T4 into T3 during inflammation. Specific cytokines inhibit the enzyme expression, like interleukin (IL)-1 and tumor necrosis factor17.
The decrease in T3 level is expected in CKD patients due to decreased conversion of peripheral deiodinase from T4 to T3. This effect is caused by metabolic acidosis and protein malnutrition, both present in CKD18. In response to the feedback inhibition of T3 and T4, the pituitary gland produces TSH, levels of which are decreased in CKD patients due to poor responsiveness to TSH, decreased renal clearance of TSH, poor responses to thyrotropin-releasing hormone, and decreased responsiveness of TRH. This can be caused by non-thyroid disease, which returns to normal after resolution of CKD19.
Thyroid hormones are significant determinants of health; according to the confidence interval (CI) 95% criterion for the disease-free residents, normal thyroid function is defined. These standard laboratory reference intervals are widely applied in research and clinical practice regardless of age. Thyroid hormones vary with age, and current reference intervals may not be appropriate for all age groups. Age is significant because age-related symptoms such as fatigue and tiredness are potential but not strongly predictive features of hypothyroidism20.
CONCLUSIONS
This study confirms a high prevalence of thyroid dysfunction in chronic kidney disease (CKD) patients due to disrupted hormone metabolism and function. The findings highlight mechanisms like decreased T3 levels and impaired protein binding, leading to different presentations of thyroid dysfunction. CKD patients are at increased risk of hypothyroidism and potentially worse cardiovascular outcomes, underlining the importance of thyroid function evaluation and management in this population.
Funding: “This research received no external funding,”
Acknowledgments: The author would like to thank the Dean of the College of Science and the Head of the Department of Biology at the College of the Science / University of Mosul for providing all the facilities to complete this research.
Conflicts of Interest: “The authors declare no conflict of interest.”
REFERENCES
1. Babić Leko, M., Gunjača, I., Pleić, N., & Zemunik, T. Environmental factors affecting thyroid-stimulating and thyroid hormone levels. Int. J.Mol. Sci.,2021: 22(12), 6521.
2. Delles, C., & Vanholder, R. Chronic kidney disease. Cli. Sci.,2017: 131(3), 225-226.
3. Pinho, N. A. D., Silva, G. V. D., & Pierin, A. M. G. Prevalence and factors associated with chronic kidney disease among hospitalized patients in a university hospital in the city of São Paulo, SP, Brazil. Braz. J. Neph.,2015: 37, 91-97.
4. Narasaki, Y., Sohn, P., & Rhee, C. M. The interplay between thyroid dysfunction and kidney disease. In Seminars in nephrology.,2021:(Vol. 41, No. 2, pp. 133-143). WB Saunders.
5. Rhee, C. M., & Kalim, S.Thyroid Status in Chronic Renal Failure Patients. In Textbook of Nephro-Endocrinology.,2018: (pp.477-492). Academic Press.
6. George, D., & Mallery, P. IBM SPSS statistics 27 step by step: A Simple Guide and reference..,2021:Routledge.
7. Keunmoe, P., Halle, M. P., Nguedia, J. C., Njounendou, A. J., Tengen, J., & Ngowe, M. The spectrum of thyroid function abnormalities and associated biochemical factors in patients with chronic kidney disease in Cameroon. Am J Biomed Sci Res.,2020:8(5), 387-396.
8. Iglesias, P., Bajo, M. A., Selgas, R., & Díez, J. J. Thyroid dysfunction and kidney disease: an update. Revi. Endocr. and Metabol. Diso.,2017: 18, 131-144.
9. Pan, B., Du, X., Zhang, H., Hua, X., Wan, X., & Cao, C. Relationships of chronic kidney disease and thyroid dysfunction in non-dialysis patients: A pilot study. Kidney and Blood Pre. Res.,2019: 44(2), 170-178.
10. Schultheiss, U. T., Daya, N., Grams, M. E., Seufert, J., Steffes, M., Coresh, J., ... & Köttgen, A. Thyroid function, reduced kidney function and incident chronic kidney disease in a community-based population: the Atherosclerosis Risk in Communities study. Nephrology Dialysis Transplantation.,2017:32(11), 1874-1881.
11. Moon, S., Kim, M. J., Yu, J. M., Yoo, H. J., & Park, Y. J. Subclinical hypothyroidism and the risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. Thyroid,2018:28(9), 1101-1110.
12. Khatiwada, S., Kc, R., Gautam, S., Lamsal, M., & Baral, N. Thyroid dysfunction and dyslipidemia in chronic kidney disease patients. BMC endocrine disorders,2015:15(1), 1-7.
13. Sharma, S. K., Dhakal, S., Thapa, L., Ghimire, A., Tamrakar, R., Chaudhary, S., ... & Lamsal, M. Community-based screening for chronic kidney disease, hypertension and diabetes in Dharan. J. Nep. Med. Assoc.,2013:52(189), 205-212.
14. Paudel, K.Prevalence and clinical characteristics of hypothyroidism in a population undergoing maintenance hemodialysis. JCDR,2014: 8(4), MC01.
15. Salih, S. S., & Yenzeel, J. H. Evaluation of Thyroid Hormones and Some Biochemical Variables in Patients with Chronic Kidney Disease. IRQ. J. Sci.,2020:985-992.
16. Bajaj, S., Purwar, N., Gupta, A., Gupta, P., & Srivastava, A.Prevalence of hypothyroidism in nondiabetic chronic kidney disease and effect of thyroxine replacement on estimated glomerular filtration rate. Ind. J. nephron.,2017: 27(2), 104.
17. Fan, J., Yan, P., Wang, Y., Shen, B., Ding, F., & Liu, Y.Prevalence and clinical significance of low T3 syndrome in non-dialysis patients with chronic kidney disease. Medical science monitor: Int. Med.J. exper. And clin.Res.2016:22,1171.
18. Rhee, C. M. The interaction between thyroid and kidney disease: an overview of the evidence. Current opinion in endocrinology, diabetes, and obesity,2016: 23(5), 407.
19. Praw, S. S., Way, J. S. A., & Weiss, R. Evaluating Thyroid Function Tests in Patients with Kidney Disease. Endocrine Disorders in Kidney Disease: Diagnosis and Treatment,2019:85-96.
20. Taylor, P. N., Lansdown, A., Witczak, J., Khan, R., Rees, A., Dayan, C. M., & Okosieme, O.Age-related variation in thyroid function–a narrative review highlighting essential implications for research and clinical practice. Thyroid Research,2023:16(1),1-12.
Received: October 9th 2023/ Accepted: January 15th 2024 / Published:15 February 2024
Citation: Jadaan T. M., Al-Hayali H. L. Chronic Kidney Disease Effect Thyroid Hormones. Revis Bionatura 2024; 1 (1) 34. http://dx.doi.org/10.21931/BJ/2024.01.01.34
Additional information Correspondence should be addressed to [email protected]
Peer review information. Bionatura thanks anonymous reviewer(s) for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
All articles published by Bionatura Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Publisher’s Note: Bionatura stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2024 by the authors. They were submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
