2024..01.01.51
Files > Conference Series > 2024 > Chimborazo
Root-shoot ratio and its relationships with physiological characteristics, growth and biomass yield of Gynura procumbens under different shade levels and plant density
Omar Ali Ahmed1,2, Martini Mohammad Yusoff1*, Azizah Misran1, Puteri Edaroyati Megat Wahab1and Qusay Abdualhamza Muttaleb1,3*
1Department of Crop Science, Faculty of Agriculture, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia.
2Department of Crop Science, Faculty of Agriculture, University of Diyala, Iraq. ORCID
3Technical Institute of Babylon, Al-Furat Al-Awsat Technical University (ATU), Iraq. ORCID
* Correspondence: [email protected]
Available from. http://dx.doi.org/10.21931/BJ/2024.01.01.52
ABSTRACT
Gynura procumbens is one of the most common medicinal plants in the Asteraceae family and has extensive pharmacological properties. The experiment was conducted to evaluate the effects of different shade levels (0 and 30% shade) and plant density (9, 15, and 25 plants m-2) on root-shoot ratio and it is a relationship with physiology, growth, and biomass yield using split-plot design with three replications. Increasing shade level to 30% shade significantly decreased root-shoot ratio (RSR) by22.54%, while total leaf dry weight per plant (TLDW) and total leaf dry weight per square meter (TLDW m-2) increased by 35.64, 11.58, and 32.18%, respectively due to negative correlation with RSR. Increasing plant density from 9 to 25 plants m-2 significantly increased RSR and TLDW m-2 by 67.71 and 18.54%, respectively, while TLDW decreased by 57.31%. There was a negative correlation between RSR and biomass yield per plant. Under stressed conditions (full sunlight and high plant density), G. procumbent plants appeared to change their strategy to absorb limited resources, allocate more biomass to the root system, and reduce the size of the aboveground parts to survive, resulting in high RSR.
Keywords: Gynura procumbens, shade, plant density, root-shoot ratio, physiology, growth, biomass.
INTRODUCTION
The Gynura procumbens is an evergreen medicinal shrub belonging to the Asteraceae family. It is widely distributed in Africa 1,2 and tropical regions of Southeast Asia and China 3,4. Non-toxic leaves have extensive pharmacological properties, including anti-diabetic, anti-hypertensive, antioxidant, and vasorelaxant activity 5. The medicinal benefits of G. procumbens are related to its bioactive compounds, such as saponins, flavonoids, and terpenoids4,6.
The root-shoot ratio (RSR) is the ratio of the belowground biomass to the biomass of the aerial part reflected in plant biomass allocation. The differences in biomass allocation between the shoot and root systems would result in variations in the RSR ratio. The decrease in biomass under biotic and abiotic stress conditions is associated with the availability of resources above and belowground, thereby resulting in trade-offs in biomass allocation between aboveground and belowground biomasses, suggesting that plants allocate biomass to acquire the most limiting resources 7,8,9,10. Many plants would change their RSR to respond to shading and low nutrient availability 11,12. Therefore, RSR is vital in studying competitive interactions between growth conditions 13.
Biomass allocation between above and belowground parts results from long-term environmental modification, significantly affecting plant growth and reproduction 14,15,16. The root biomass was less affected by biomass allocation under stress conditions than shoot biomass, reflected in the plant RSR ratio 17. The differences in RSR due to the differences in above and belowground biomass indicate that different biomass exhibits various biomass allocation strategies. RSR for grass (4.23) was significantly higher than that of shrubs (1.68) and trees (0.40), and evergreen plants had higher RSR than deciduous plants in tropical rainforests 17.
Generally, the response of plants to light intensity depends on the type of plant, resulting in different concentrations of plant secondary metabolites 18. Consequently, the medicinal properties of the target plants are affected, which also brings about changes in the morphology and physiology of the plants in question 19,20. Changes in microclimate and light intensity due to shading of the canopy resulting from different plant densities also affected above and belowground biomass 21.
Plant density or plant canopy is also a critical agronomic practice for effectively capturing environmental resources such as solar radiation, water, and nutrients 22, which can enhance optimum plant population 23 and influence the physiological and phytochemical characteristics of the plants. An increasing number of plants per unit area led to increased interplant competition for resources, resulting in the depletion of some limited resources. Plants allocated more biomass to roots to absorb more water and nutrients for survival and acclimatization under high competition or stress conditions 24,13. Moreover, the competition for resources led to a change in biomass allocation in the plant, which resulted in a variation in RSR 25,26.
Therefore, understanding the effects of different environmental conditions on the above- and below ground biomass of G. procumbent plants through RSR helps improve the quality and quantity of medicinal plants to meet the high demand. Therefore, the present study was conducted to determine the RSR and its relationship to growth, physiological attributes, and biomass yield under different shade levels and plant densities of G. procumbens.
MATERIALS AND METHODS
Experimental design and treatments
The experiment was conducted in November 2017 in a split-plot design (main plots presented by shade levels; subplots presented by plant densities) with three replicates using wooden planting boxes (2 m × 1 m × 0.5 m). The two levels of shade used were 0 and 30% using custom-made polyethylene shade netting with three plant densities (9, 15 and 25 plants m-2) using 9 plants m-2 = 33.3 × 33.3 cm, 15 plants m-2 = 20 × 33.3 cm and 25 plants m-2 = 20 × 20 cm, respectively were implemented.
Root: Shoot Ratio (RSR)
Root: shoot ratio (belowground biomass to aboveground biomass) for each treatment was determined according to the following formula:
RSR = Total root dry weight / Total shoot dry weight
Net Photosynthesis Rate and Total Chlorophyll Content
A portable photosynthesis system (LICOR-64001 LI-COR Inc., USA) measured the net photosynthesis rate between 0900 and 1100 hours.
Total leaf chlorophyl1 content was also measured using a modified method of Lichtentaler and Wellburn (1983). Leaf weighing 0.2 g were collected from plant samples and stored in small plastic vials containing 20 mL of 80% (v/v) acetone. Absorbance was measured using a scanning spectrometer Model UV 3101 PC, and the total chlorophyll was calculated as the sum of chlorophyll a and chlorophyll b using the following.
Chlorophyll a = 11.75 (Absorbance 662) − 2.350 (Absorbance 645)
Chlorophyll b = 18.61 (Absorbance 645) − 3.960 (Absorbance 662)
Leaf area and Leaf area index
A leaf area meter (LI-3100 Area Meter, USA) measured the total leaf area of all harvested leaves (TLA, cm2). The leaf area index (LAI) for each sample was computed after measuring the TLA and area of each plant using the following formula:
LAI = TLA (cm2) / Soil area (cm2)
Biomass dry weight
The plants were harvested to measure the biomass yield of leaves per plant and square meter. Three plants per treatment were harvested. Fresh samples were oven-dried at 45 °C until a constant weight of dry samples was achieved. An electronic balance (BP 2100, Sartorius, Germany) was used to determine the total dry leaf weight per plant and the dry leaf weight per square meter.
Crop growth rate (CGR)
It was measured using the formula, CGR = (W2 – W1) / (t2 – t1), where W1= total dry weight of plant at time 1 (t1) and W2= total dry weight of plant at time 2 (t2).
Statistical data analysis
Data collected were subjected to statistical analysis of variance (ANOVA) for split-plot design using the SAS program (SAS version 9.4, Car, NC) to determine the statistical significance of main and interaction effects. Significant main effects means were separated using Least Significant Differences (LSD) (P≤0.05). Only the main effect comparisons were performed if an interaction between shade levels and plant density was insignificant. The relationship between parameters was determined using correlation analysis.
RESULTS
Root-shoot ratio (RSR)
Shade and plant density significantly affected the root-shoot ratio (RSR). The RSR of G. procumbens decreased by 30.09% with increasing shade levels from 0 to 30% (Figures 1-A). The percentage of increment of RSR at high plant density was 67.71 and 33.33 % compared with 9 and 15 plant m-2, respectively (Figures 1-B).
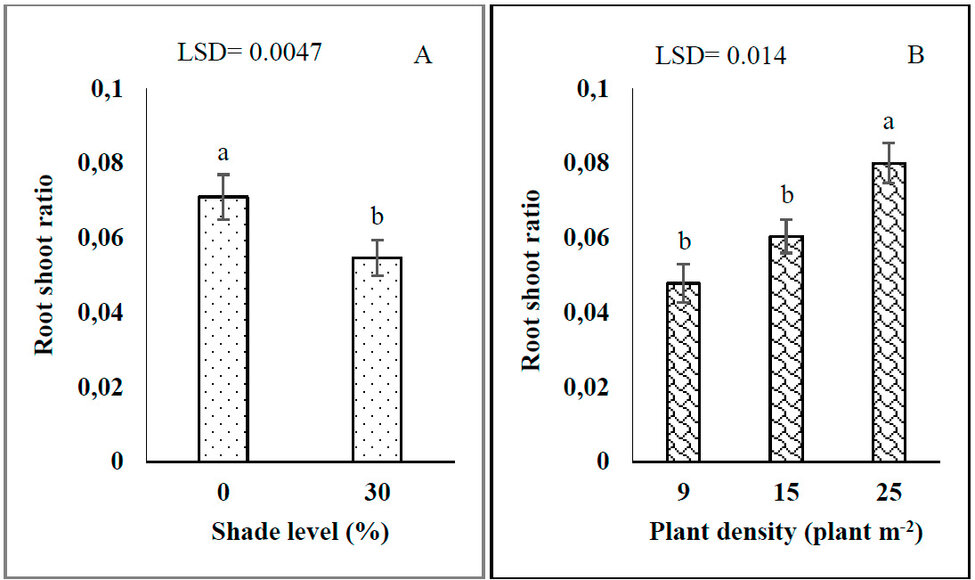
Figure 1: Effects of shade levels (A) and plant density (B) on G. procumbens root-shoot ratio.
Significant interaction effects of plant density and plant age on the RSR of G. procumbent plants (Figure 6.2). The high value of RSR was 0.134 under all plant densities at 0 DAT. Regardless of plant density, the RSR decreased to 72.38% with increasing plant age from 0 to 30 days after transplanting. At 30 days after transplanting, the RSR increased by 27.4% at low plant density (9 plants m-2) than at high plant density (25 plants m-2). On the other hand, at 60 DAT, the high plant density (25 plants m-2) increases RSR by (42.45 and 33.34%) more than both 9 and 15 plants m-2. The percentage of increment in RSR at high plant density (25 plants m-2) at 60 DAT was 52.0% as compared with 30. In addition, the RSR increased with increased plant density and plan age from 60 to 90 DAT. At 90 DAT, the RSR increased by 67.39% at high plant density (25 plants m-2) compared with low plant density (9 plants m-2). The result demonstrated no significant differences between 30, 60 and 90 DAT on RSR under low plant density. At the same time, the result showed that the RSR, after 30 days of transplanting, started to increase with increasing plant age under both 15 and 25 plants m-2 (Figure 6.2).
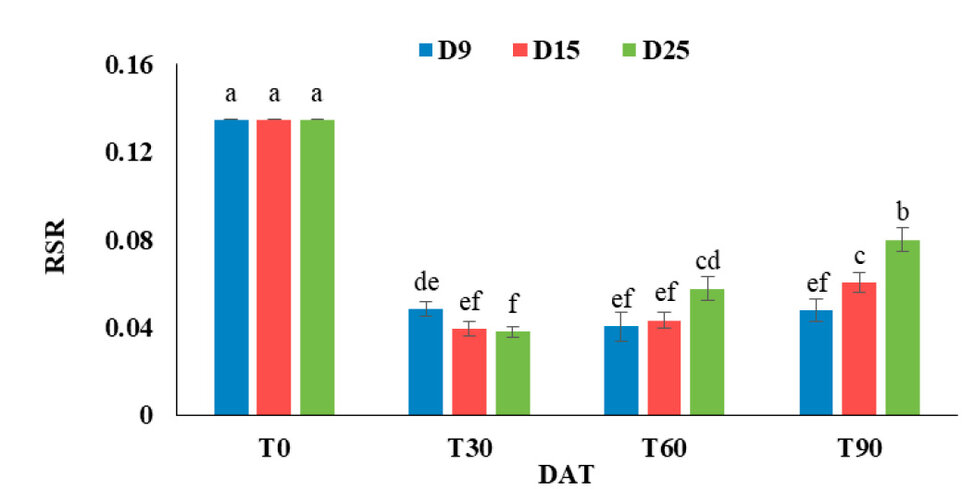
Figure 2: Effect of plant density and plant age on G. procumbens root shoot ratio.
Net photosynthesis rate (PN)
Different shade levels and plant densities had significant effects on the net photosynthesis rate (PN) (P≤0.05) (Table 1). Thirty percent shade level increased net photosynthesis rate by 12.16% in comparison with zero level of shade. In contrast, G. procumbens' net photosynthesis rate decreased markedly to 20.12% when planted at 25 plants m-2, whereas a density of 9 plants m-2 resulted in the highest net photosynthesis rate. According to the correlation result, the net photosynthesis rate response to the studying factors was the opposite of the RSR response (Table 4).
Total chlorophyll (a+b)
Shade and plant density had significant effects (P≤0.05) on total chlorophyll content Chl (a+b) (Table.1). High shade level of 30% showed higher total chlorophyll Chl (a+b) (19.30 mg g-¹ FW). In contrast, lower shade levels recorded the lowest total chlorophyll (16.85 mg g-¹ FW). Higher plant density of 25 plants m-2 displayed lower total chlorophyll content Chl (a+b) (16.61 mg g-¹ FW) in comparison with control (19.81 mg g-¹ FW) of 9 plants m-2. The increase in total chlorophyll content in the G. procumbens plant was accompanied by a decrease in RSR due to the result of the coefficient of variation analysis (Table 4).
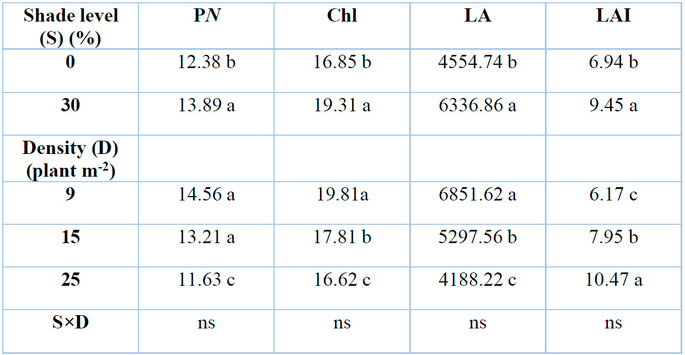
Means with the same letters in the same columns are not significantly different at p ˂ 0.05 (LSD); ns = insignificant.
Table 1: Effects of shade levels and plant density on G. procumbens net photosynthesis rate (PN), total chlorophyll content (Chl), leaf area (LA) and leaf area index (LAI).
Leaf area (LA)
Shade levels and plant density had significant effects (P≤0.05) on the leaf area of G. procumbens plants (Table 1). The result showed shade effects were more visible on leaf area (6336.86 cm2) when plants were grown under 30% shade level. However, under full sunlight (0% shade), plants had leaf area at the lowest level (4554.71 cm2). The leaf area increased by 39.12% under 30% shade compared to 0%. On the other hand, low plant density (9 plants m-2) had higher leaf area (6851.62 cm2) than 15 plants m-2 (5297.56 cm2) and 25 plants m-2 (4188.23 cm2) densities, as shown in Table 2. Due to their strong negative correlation, there was an inverse relation between leaf area and RSR in G. procumbens (Table .4).
Leaf area index (LAI)
Shade level and plant density significantly affected the leaf index of G. procumbens (Table 1). There was an increase in leaf area index under 30% shade level by 36.16% compared with the control 0% shade. The results showed a linear increase in leaf area index with an increase in the number of plants grown per unit area. It increased around 28.85 and 69.69% for 15 and 25 plants grown per square meter, respectively, compared to 9 plants m-2. There was no correlation between LAI and RSR in G. procumbens (Table 6.4).
Fresh and dry weight
Total leaf fresh weight per plant (TLFW)
Total leaf fresh weight per plant was significantly affected by different levels of shade and total number of plants growing per unit area (plant density) (P≤0.05) as presented in (Table.2). Higher level of shade (30%) linearly increased leaf fresh weight of G. procumbens by 32.39% plant-1 in relation to control (0% shade). On the contrary, plant density decreased total leaf fresh weight from 26.54 to 40.75% as plant density increased from 15 to 25 plants m-2 compared to 9 plants m-2. A strong negative correlation existed between total leaf fresh weight per plant and RSR in G. procumbens (Table 6.4).
Total leaf fresh weight per square meter (TLFW m-2)
Shade levels and plant density had significant effects on leaf fresh weight per unit area (P≤0.05) (Table 2). The results revealed that the total leaf fresh weight of G. procumbens per square meter increased as shade level and plant density increased. The corresponding increase values were 26.8% for a 30% level of shade compared to 0% shade and 22.44 and 64.55% for a plant density of 15 and 25 plants m-2 compared to 9 plants m-2, respectively.
Total fresh weight per plant (TFW)
Shading and plant density had significant effects (P≤0.05) on total fresh weight per plant (Table 2). There was an increase of 37.64% with an increase in shade level from 0 to 30%. Results showed that total fresh weight per plant considerably decreased with the increase of plant density from 9 to 15 and 25 plants m-2 densities. The total fresh weight of plants decreased by 26.47 and 41.95% at 15 and 25 plants m-2 than 9 plants m-2, respectively. The total fresh weight per plant inversely responded to shade level and plant density more than RSR due to their strong negative correlation (Table 4).
Total fresh weight per square meter (TFW m-2)
Total fresh weight per square meter was significantly affected by different levels of shading and plant density (Table 2). Total fresh weight increased by 33.71% when G. procumbens plants were subjected to 30% shade than complete sun treatment. Consequently, the same parameter increased by 22.55 and 61.25% at 15 and 25 plants m-2, respectively, as compared with low plant density (9 plants m-2).
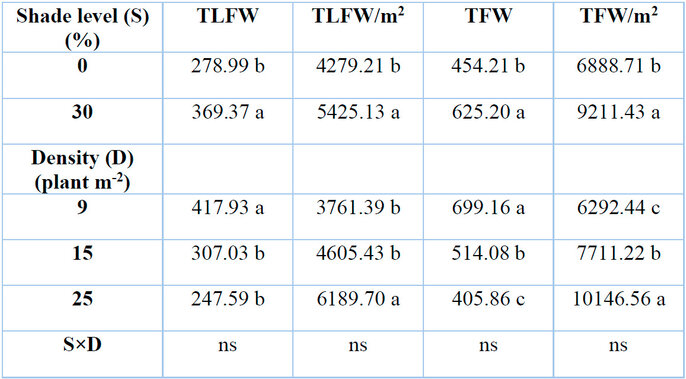
Means with the same letters in the same columns are not significantly different at p ˂ 0.05 (LSD); ns = not significant.
Table 2: Effects of shade levels and plant density on G. procumbens total leaf fresh weight (TLFW), total leaf fresh weight per square meter (TLFW/m2), total fresh weight (TFW), and total fresh weight per square meter (TLW/m2).
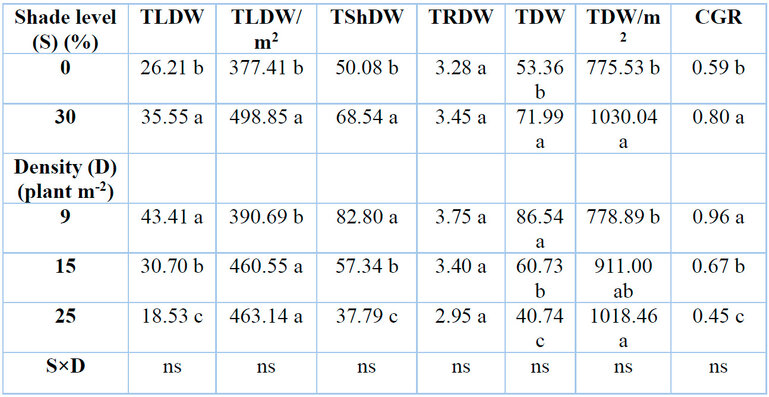
Total leaf dry weight per plant (TLDW)
Total leaf dry weight per plant was significantly affected by different levels of shading and plant density (Table 3). level of 30% shade resulted in higher total leaf dry weight per plant than under control, with a percentage increase of 35.63%. In contrast, the total leaf dry weight per plant decreased with an increase in plant density. The reductions were 29.28 and 57.31% when G. procumbens plants were grown at 15 and 25 plants m-2 densities compared with 9 plants m-2. Due to their strong negative correlation, there was an inverse relationship between total leaf dry weight per plant and RSR in G. procumbens (Table .4).
Total leaf dry weight per square meter (TLDW m-2)
Different shade levels and plant density significantly affected total leaf dry weight per square meter (Table 3). Total leaf dry weight per square meter increased by 32.17% under 30% shade than under control. In contrast, the total leaf dry weight of G. procumbent plant per square meter increased by 18.54% with an increase in plant density from 9 to 25 plants m-2, without any differences between 25 and 15 plant m-2.
Total dry weight per plant (TDW)
Different shade levels and plant densities significantly affected the total dry weight per plant for G. procumbens (Table 3). The result shows an increase in total dry weight per plant by 34.92% under 30% shade compared to 0%. Conversely to the above results, higher plant densities of 15 and 25 plants m-2 reduced total dry weight by 29.82 and 52.92% compared with low plant densities of 9 plants per square meter. The total dry weight per plant in G. procumbens was inversely related to studying factors other than RSR due to their strong negative correlation (Table 4).
Means with the same letters in the same columns are not significantly different at p ˂ 0.05 (LSD); ns = not significant.
Table 3: Effects of shade levels and plant density on G. procumbens total leaf dry weight (TLDW), total leaf dry weight per square meter (TLDW/m2), total shoot dry weight (TShDW), total root dry weight (TRDW), total dry weight (TDW), total dry weight per square meter (TDW/m2), and crop growth rate (CGR).
Total dry weight per square meter (TDW m-2)
Shading and plant density significantly affected the total dry weight per square meter (TDW) of G, procumbens (Table 3). Shading of 30% recorded higher total dry weight per square meter compared to 0% shade when it increased by 32.81% compared to full sunlight (0% shade). Total dry weight per square meter rose with the increase in plant density. The higher plant density of 25 plants m-2 recorded a higher total dry weight per square meter of 30.75% compared with 9 plants m-2. No correlation existed between total dry weight per square meter of G, procumbens and RSR (Table .4).
Crop growth rate (CGR)
Crop growth rate (CGR) showed high effects by shading and the number of plants per unit area (Table 3). The crop growth rate increased by 35.06% under a 30% level of shade. Density at 9 plants m-2 reported a higher CGR value (0.959 g day-1) than (0.67 and 0.45 g day-1) at density levels of 15 and 25 plants m-2, respectively. There was an inverse relationship between crop growth rate and RSR (Table .4).
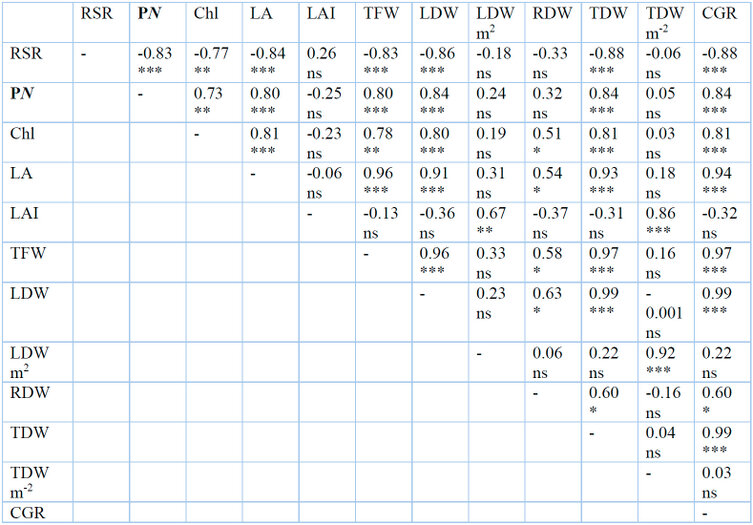
Table 4: Correlation coefficient (r) between photosynthesis rate (PN), chlorophyll content a+b (Chl), leaf area (LA), leaf area index (LAI), total fresh weight (TFW), total leaf dry weight (TLDW), total leaf dry weight per square meter (TLDW m-2), total root dry weight (TRDW), total dry weight (TDW), total dry weight per square meter (TDW m-2), and crop growth rate (CGR) of G. procumbens under different shade levels and plant density. Ns, insignificant. *, significant at P˂0.05, **, P˂0.01. ***, P˂0.001
DISCUSSION
The root-shoot ratio reflects the differences in biomass allocation between aboveground and belowground associated with climatic conditions, resulting in root/shoot competition and cooperation about resources for adaptation and survival in variable and complex conditions27,28. The shoot-root system plays an essential role in plant development and productivity, the photosynthetic process, and, finally, the growth and maintenance of roots29-33.
The reduction in total root dry weight TRDW by 21.33% with increasing plant density from (9 to 25 plants m-2) (Table. 3), while total shoot dry weight TShDW was reduced by 54.35%, which proved that roots (belowground part) were less affected by study factors than shoot (aboveground part) which resulted in increased RSR due to inverse relationship between RSR and TShDW (aboveground part). On the other hand, increasing the shade level from full sunlight (0% shade) to 30% shade increased the aboveground part (TShDW) by 36.86%; however, the belowground part (root system) increased by 5.18% only, which demonstrated that under optimum light condition, G. procumbens plants allocated more biomass to aerial part than root. Improvement in wheat grain yield was associated with the dry matter distribution ratio between aerial parts and the root system under drought conditions34. Root systems consume double assimilation products than aerial parts36. The results demonstrated that the root (belowground part) of G. procumbent plants was less affected by shade level and plant density.
The high values of RSR of G. procumbens under high plant density are in agreement with Ericsson36, who reported that high competition of resources between plants under high plant density resulted in high RSR due to differences in dry matter allocation when the low nutrient resource inhibits plant growth. Similarly, the results of 37 and 38 demonstrated that the root-shoot ratio of plants growing in variable patchy soil nutrient conditions was smaller than those in stable soil nutrient conditions, which promoted more fine root growth than a variable one. Dolt39 et al. elucidated that the increase in biomass of B. erectus under high density was superior in exploiting surplus light and the increase in available belowground resources.
Similar findings were reported by Martins40, who illustrated the shoot contribution to root growth even when shoot growth is inhibited due to a severe reduction in shoot development without any effect on root growth on tomato plants. Under limited exogenous resources, plants allocate more biomass to those organs that absorb the scarcest resources28. Biomass allocation between above- and belowground parts depends on adjusting C allocation43, which results from long-term modification to the environmental conditions, which has critical effects on plant growth and reproduction14-16.
Accordingly, increasing allocation to underground organs such as fine roots in place of aboveground parts indicates the growing relative importance of limiting soil resources, i.e., nutrients and water42. The root-shoot ratio increased with increasing plant density and decreased with increasing aerial biomass, stand age, and volume 34.
Higher reduction in TShDW than TRDW of G. procumbens plants, which resulted in high RSR, supported the optimal partitioning theory that referred to trade-offs in biomass allocation between aboveground and belowground parts to acquire the most limiting resources7-10. According to Qi et al.13, the various environmental conditions proved the optimal partitioning theory that the more significant growth and photosynthetic rates for deciduous plants than evergreen plants, RSR of evergreen plants (angiosperms) was significantly higher than deciduous plants (gymnosperms).
As plants grow larger and higher, they allocate more biomass to the aboveground components. However, more biomass yield would be needed in the root systems to acquire more water and nutrients for survival and acclimatization under different stress conditions. The differences in RSR of G. procumbent plants at different plant ages illustrated that in the first stage, the roots grew faster, and the plants allocated more biomass to the roots, resulting in the high value of RSR to stabilize the plants and absorb water and elements. Meanwhile, RSR, after 30 days of transplanting, started to grow slowly. This result parallels Qi et al.13's findings, which reported that plants allocated more biomass to roots in the seedling stage or due to climatic conditions, soil fertility, and management practices5,43,44.
Results of the present experiments showed that the highest net photosynthetic rate of G. procumbens was 13.89 µmol m-2 s-1 at 30% shade than 12.38 µmol m-2 s-1 at 0% shade (full sunlight) (Table 1). Net photosynthetic was negatively affected by excessive light under an open field due to photoinhibition45.
Increasing plant density from 9 to 25 plants m-2 decreased the net photosynthetic rate by 20.12% (Table 1). Competition among plants increases with an increasing number of plants per unit area. These results agree with 47, which reported that a reduction in the photosynthetic rate of blessed thistle was associated with nitrogen reduction under high plant density. Hence, the net photosynthetic rate of G. procumbent plants decreased under full sunlight but at higher plant density.
The present study revealed a significant negative correlation (r = -0.83) between RSR and PN (Table 4). Under high light intensity (full sunlight) and high plant density, the net photosynthetic rate of G. procumbens is reduced as the plant responds to excessive light. Meanwhile, RSR increased under the same conditions because the root system was less affected by stress than aboveground parts or plants, which allocated more biomass to the root than the shoot. For survival and multiplication, plants have to modify their distribution of photosynthetic to different organs for adaptation as it could impair other plants' functions such as photosynthesis, nutrient uptake, and growth48. A similar observation was also reported on wheat34. The findings align with 33,49, who reported that crops with higher grain yields from partitioning more dry matter to aboveground parts have lower RSR. A high photosynthetic rate offsets the low RSR under low plant density. A low photosynthetic rate was recorded under high plant density, which yielded high RSR, as revealed in Compositae plants50.
Increase chlorophyll content Chl (a+b) by increasing shade levels from full sunlight to 30%. It significantly decreased with increasing plant density from 9 to 25 plants m-2. The percentage of increments of Chl a+b was 14.54%, with increasing shade levels from 0 to 30% shade. It decreased by 16.15% with increasing plant density from 9 to 25 plants m-2. The decrease in total chlorophyll content under full sunlight compared to 30% shade may be related to chlorophyll pigment impairment due to absorbing excessive light energy51.
On the other hand, shade and low light conditions caused chlorophyll content to increase as a physiological response to low light environment. This maximized available light utilization by channeling more resources into chlorophyll synthesis51,52. The plants regulated their chlorophyll synthesis to absorb more solar radiation for photosynthesis, and changes in irradiance level may elicit physiological responses at the level of leaf and chloroplast53. In addition, the low total chlorophyll content of G. procumbens under high plant density may be due to interplant competition for resources under high plant density. In the present study, an increase in plant density resulted in decreased chlorophyll content of high plant density, which led to the depletion of some resources and nutrients, especially nitrogen 47,54. The results of the present experiment agree with those of other researchers47,55,56.
There was a significant negative correlation (r = - 0.77) between RSR and total chlorophyll content (Table .4). The inverse relationship between them means that under stress conditions (high light intensity and plant density), G. procumbent plants allocated more biomass to root despite the low chlorophyll content. Decreased chlorophyll pigments as a response to full sunlight and a high number of plants per square meter to survive was offset by adverse response in the root system to water and nutrient supply. The findings in the present experiment align with results reported61, which explained that decreased RSR was associated with high yield due to high photosynthetic rate and chlorophyll content, which accelerated the growth and development of aboveground parts.
Leaf area (LA) and leaf area index (LAI) were highly affected by shade levels and plant density. The treatment of 30% shade resulted in higher LA (6336.86 cm2) and LAI (9.45) than the lowest under full sunlight. G. procumbens plants recorded the highest LA (6851.62 cm2) at low plant density than (4188.23 cm2) recorded at high plant density (25 plants m-2). At the same time, the highest LAI was (10.47) at high plant density than (6.17) at 9 plants m-2. The results suggested that to absorb a high amount of light under low light conditions, G. procumbent plants increased leaf size as a physiological response. The results agree with those of other studies 58,59,60, which reported that plants under low light conditions maximize available leaf surface area by producing bigger and thinner leaves for interception of limited light incident. Also, wider intra-row spacing (45 cm) resulted in higher LA than (15 cm) in Thymus vulgaris L61. The increasing plant density from 20 to 25 plants m-2 led to a higher LAI of soybean66. G. procumbens plants responded to low irradiance by increasing LAI to increase light penetration within the canopy and absorb more light, resulting in higher biomass per unit area.
There was a significant negative correlation (r = -0.84) between LA and RSR, while there was no significant correlation (r = 0.26) between LAI and RSR (Table .4). The results showed that plants under stressed conditions modified size and biomass allocated between shoot and root system in order to survive. Leaf area decreased under full sunlight and high plant density, resulting in increased RSR due to a reduction in plant size compared to the root system. This reduction in LA was probably due to decreased leaf growth and expansion under stress conditions with nutrient deficiency42,63,64.
The G. procumbens total leaf dry weight (TLDW) and total dry weight (TDW) per plant increased by 35.63 and 34.92% as the shade level increased from full sunlight to 30% shade. Also, total leaf dry weight per square meter (TLDW m-2) and total dry weight per square meter (TDW m-2) increased by 32.17 and 32.81% under 30% shade compared to 0% shade. At the same time, the low number of plants per square meter (9 plants m-2) resulted in higher TLDW and TDW g per plant, which were 43.41 and 86.54 g plant-1, respectively. However, the higher TLDW m-2 and TDW m-2 were 463.14 and 1018.46 g m-2 at low plant density (25 plants m-2).
The experiments showed a significant positive correlation between TLDW per plant and PN and LA (r = 0.84 and 0.92), respectively (Table 4). The results indicated higher biomass yield in individual plant under 30% shade and low plant density (9 plants m-2) related to high photosynthesis rate and leaf area which resulted in high CGR due to the significant positive correlation between both CGR with PN and LA (r = 0.84 and 0.94) (Table.4). When there were no stress conditions, the high LA and PN of G. procumbens resulted in high biomass per plant; however, under stress condition (high plant density) high biomass per square meter associated with high LAI. These results agree with the finding of 24,65,66, which reported that optimal plant density enhances using resources (radiation, water, and nutrients) due to optimal LAI. However, decreased biomass in individual plants under high plant density was related to high competition for resources, resulting in low LA and low photosynthetic capacity.
The higher dry biomass yield per square meter under 30% shade and high plant density (25 plants m-2) was associated with a significant positive correlation with LAI only (r = 0.86) (Table 4). This result indicated that the high biomass yield per square meter under 30% shade and high plant density was associated with high LAI. Shading conditions resulting from net shade or canopy under high-density produce higher biomass than open field conditions. The increasing water evaporation, canopy senescence, and increase in maintenance respiration in open fields than shade due to warmer temperatures led to a decrease in final biomass67. High plant density was a sustainable strategy to improve rice yield compared to low plant density, under full sunlight and 30% shade67. The condition that results from an increasing number of plants per unit area is favorable for producing a high biomass of G. procumbens.
The present results agree with several other previous studies57,59,68. There was a significant negative correlation (r = -0.83, - 0.89, and – 0.88) between (TFW, TShDW, and TDW) with RSR, respectively (Table 4). The increase in shade level from 0 to 30% increased TFW, TShDW, and TDW by 37.64, 36.86, and 34.92%, while RSR decreased by 23.13%. On the other hand, an increasing the number of plants per unit area from 9 to 25 plants m-2 resulted in decreased TFW, TShDW, and TDW by 41.95, 54.35, and 52.92%, but RSR increased by 67.71%. Under 30% shade and low plant density (9 plants m-2), G. procumbens plants allocated more biomass to the aerial part than root. In contrast, with increasing light intensity (no shade) and the high number of plants per square meter, plants allocated more biomass to root as a physiological response to stress conditions to survive. The contrast between biomass yield and RSR is associated with a negative relationship between RSR and (photosynthetic rate, LA, and number of branches).
CONCLUSIONS
The current study has demonstrated that the higher G. procumbens biomass per plant, which negatively correlated with RSR, was under 30% shade and 9 plants per square meter due to more biomass allocated to the aboveground part at 30% shade and low plant density. Meanwhile, under full sunlight and high plant density, G. procumbens plants allocated more biomass to belowground parts, which resulted in low biomass and high RSR value. Low biomass yield, high RSR under full sunlight (0 % shade), and high plant density are associated with low photosynthesis rate, chlorophyll content, and leaf area—higher biomass per unit area under 25 plants per square meter is associated with high LAI. Under stress conditions, G. procumbent plants change their biomass allocation strategy to absorb limited resources due to surviving by the reduced size of aboveground parts rather than root, which resulted in high RSR.
REFERENCES
1. Rahman, A., & Al Asad, M. S. (2013). Chemical and biological investigations of the leaves of Gynura procumbens. International Journal of Biosciences, 3(4), 36–43.
2. Sukadeetad, K., Nakbanpote, W., Heinrich, M., & Nuengchamnong, N. (2018). Effect of drying methods and solvent extraction on the phenolic compounds of Gynura pseudochina (L.) DC. Leaf extracts and their anti-psoriatic property. Industrial Crops and Products, 120, 34–46.
3. Li, X., Schmid, B., Wang, F., & Paine, C. E. T. (2016). Net assimilation rate determines the growth rates of 14 species of subtropical forest trees. PloS One, 11(3), e0150644.
4. Mou, P., Jones, R. H., Tan, Z., Bao, Z., & Chen, H. (2013). Morphological and physiological plasticity of plant roots when nutrients are both spatially and temporally heterogeneous. Plant and Soil, 364(1–2), 373–384.
5. Rohin, M. A. K., Jumli, M. N., Ridzwan, N., Baig, A. A., Latif, A. Z. A., & Hadi, N. A. (2018). Effect of Gynura procumbens extracts on anti-proliferative activity and its associated morphological changes of human Glioblastoma multiforme cell line (U-87). Pharmacognosy Journal, 10(3), 492-496.
6. Tan, H. L., Chan, K.-G., Pusparajah, P., Lee, L.H., & Goh, B.H. (2016). Gynura procumbens: an overview of the biological activities. Frontiers in Pharmacology, 7, 52
7. Hertel, D., Strecker, T., Müller‐Haubold, H., & Leuschner, C. (2013). Fine root biomass and dynamics in beech forests across a precipitation gradient–is optimal resource partitioning theory applicable to water‐limited mature trees?. Journal of Ecology, 101(5), 1183-1200.
8. Kobe, R. K., Iyer, M., & Walters, M. B. (2010). Optimal partitioning theory revisited: nonstructural carbohydrates dominate root mass responses to nitrogen. Ecology, 91(1), 166–179.
9. Tredennick, A. T., Adler, P. B., Grace, J. B., Harpole, W. S., Borer, E. T., Seabloom, E. W., Anderson, T.M., Bakker, J. D., Biederman, L. A., Brown, C. S., & Brown, C. S. (2016). Comment on Worldwide evidence of a unimodal relationship between productivity and plant species richness. Science, 351(6272), 457.
10. Ledo, A., Paul, K. I., Burslem, D. F. R. P., Ewel, J. J., Barton, C., Battaglia, M., Brookshank, K., Carter, J., Eid, T. H., England, J. R., & Fitzgerald, A. (2018). Tree size and climatic water deficit control root to shoot ratio in individual trees globally. New Phytologist, 217(1), 8–11.
11. Aphalo, P. J., Ballare, C. L., & Scopel, A. L. (1999). Plant-plant signalling, the shade-avoidance response and competition. Journal of Experimental Botany, 50(340), 1629–1634.
12. Reynolds, H. L., & D'antonio, C. (1996). The ecological significance of plasticity in root weight ratio in response to nitrogen: opinion. Plant and Soil, 185(1), 75–97.
13. Qi, Y., Wei, W., Chen, C., & Chen, L. (2019). Plant root-shoot biomass allocation over diverse biomes: A global synthesis. Global Ecology and Conservation, 18, e00606.
14. Brown, S. (2002). Measuring carbon in forests: current status and future challenges. Environmental Pollution, 116(3), 363–372.
15. Houghton, R. A. (2005). Aboveground forest biomass and the global carbon balance. Global Change Biology, 11(6), 945–958.
16. Kang, M., Ji, W., & Jiang, Y. (2012). Responses of belowground biomass and biomass allocation to environmental factors in central grassland of Inner Mongolia. Acta Agrestia Sinica, 20(2), 268–274.
17. Wang, X., Fang, J., & Zhu, B. (2008). Forest biomass and root–shoot allocation in northeast China. Forest Ecology and Management, 255(12), 4007–4020.
18. Gu, X.-D., Sun, M.-Y., Zhang, L., Fu, H.-W., Cui, L., Chen, R. Z., Zhang, D. W., & Tian, J. K. (2010). UV-B induced changes in the secondary metabolites of Morus alba L. leaves. Molecules, 15(5), 2980–2993.
19. Briskin, D. P., & Gawienowski, M. C. (2001). Differential effects of light and nitrogen on production of hypericins and leaf glands in Hypericum perforatum. Plant Physiology and Biochemistry, 39(12), 1075–1081.
20. Kurata, H., Matsumura, S., & Furusaki, S. (1997). Light irradiation causes physiological and metabolic changes for purine alkaloid production by a Coffea arabica cell suspension culture. Plant Science, 123(1–2), 197–203.
21. Dolt, C., Goverde, M., & Baur, B. (2005). Effects of experimental small-scale habitat fragmentation on above-and belowground plant biomass in calcareous grasslands. Acta Oecologica, 27(1), 49–56.
22. Singh, R. D., Meena, R. L., Singh, M. K., Kaul, V. K., Lal, B., Acharya, R., & Prasad, R. (2006). Effect of manure and plant spacing on crop growth, yield and oil-quality of Curcuma aromatica Salisb. in mid hill of western Himalaya. Industrial Crops and Products, 24(2), 105–112.
23. Rossini, M. A., Maddonni, G. A., & Otegui, M. E. (2011). Inter-plant competition for resources in maize crops grown under contrasting nitrogen supply and density: Variability in plant and ear growth. Field Crops Research, 121(3), 373–380.
24. Wang, R., Cheng, T., & Hu, L. (2015). Effect of wide–narrow row arrangement and plant density on yield and radiation use efficiency of mechanized direct-seeded canola in Central China. Field Crops Research, 172, 42-52.
25. Gersani, M., Brown, J. S., O'Brien, E. E., Maina, G. M., & Abramsky, Z. (2001). Tragedy of the commons as a result of root competition. Journal of Ecology, 89(4), 660–669.
26. Brien, E. E., Gersani, M., & Brown, J. S. (2005). Root proliferation and seed yield in response to spatial heterogeneity of belowground competition. New Phytologist, 168(2), 401–412.
27. Hermans, C., Hammond, J. P., White, P. J., & Verbruggen, N. (2006). How do plants respond to nutrient shortage by biomass allocation? Trends in Plant Science, 11(12), 610–617.
28. Marschner, H. (1995). Mineral Nutrition of Higher Plants. Second Edition Academic Press Edition London.
29. Araya, T., von Wirén, N., & Takahashi, H. (2016). CLE peptide signaling and nitrogen interactions in plant root development. Plant Molecular Biology, 91(6), 607–615.
30. Manschadi, A. M., Hammer, G. L., Christopher, J. T., & Devoil, P. (2008). Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.). Plant and Soil, 303(1–2), 115–129.
31. Osaki, M., Shinano, T., Matsumoto, M., Zheng, T., & Tadano, T. (1997). A root-shoot interaction hypothesis for high productivity of field crops. In Plant Nutrition for Sustainable Food Production and Environment (pp. 669–674). Chap, Springer.
32. Yang, C., Yang, L., Yang, Y., & Ouyang, Z. (2004). Rice root growth and nutrient uptake as influenced by organic manure in continuously and alternately flooded paddy soils. Agricultural Water Management, 70(1), 67–81.
33. Zhang, H., Xue, Y., Wang, Z., Yang, J., & Zhang, J. (2009). Morphological and physiological traits of roots and their relationships with shoot growth in "super" rice. Field Crops Research, 113(1), 31–40.
34. Zhang, X., Chen, S., Sun, H., Pei, D., & Wang, Y. (2008). Dry matter, harvest index, grain yield and water use efficiency as affected by water supply in winter wheat. Irrigation Science, 27(1), 1–10.
35. Passioura, J. B. (1983). Roots and drought resistance. In Developments in Agricultural and Water Management, 7(1-3), 265-280.
36. Ericsson, T. (1995). Growth and shoot: root ratio of seedlings in relation to nutrient availability. In Nutrient uptake and cycling in forest ecosystems (pp. 205–214). Chap, Springer.
37. Maskova, T., & Herben, T. (2018). Root: shoot ratio in developing seedlings: How seedlings change their allocation in response to seed mass and ambient nutrient supply. Ecology and Evolution, 8(14), 7143–7150.
38. Mou, K. M., & Dash, P. R. (2016). A Comprehensive Review on Gynura Procumbens Leaves. International Journal of Pharmacognosy, 3(4), 167–174.
39. Martins, A. O., Omena-Garcia, R. P., Oliveira, F. S., Silva, W. A., Hajirezaei, M.-R., Vallarino, J. G., Ribeiro, D. M., Fernie, A. R., Nunes-Nesi, A. & Araújo, W. L. (2019). Differential root and shoot responses in the metabolism of tomato plants exhibiting reduced levels of gibberellin. Environmental and Experimental Botany, 157, 331–343.
40. Leuschner, C., Moser, G., Bertsch, C., Röderstein, M., & Hertel, D. (2007). Large altitudinal increase in tree root/shoot ratio in tropical mountain forests of Ecuador. Basic and Applied Ecology, 8(3), 219–230.
41. Bolinder, M. A., Angers, D. A., Bélanger, G., Michaud, R., & Laverdière, M. R. (2002). Root biomass and shoot to root ratios of perennial forage crops in eastern Canada. Canadian Journal of Plant Science, 82(4), 731–737.
42. Bloom, A. J., Chapin, F. S., & Mooney, H. A. (1985). Resource limitation in plants--an economic analogy. Annual review of Ecology and Systematics, 363-392.
43. Sainju, U. M., Allen, B. L., Lenssen, A. W., & Ghimire, R. P. (2017). Root biomass, root/shoot ratio, and soil water content under perennial grasses with different nitrogen rates. Field Crops Research, 210, 183–191.
44. DaMatta, F. M., Ronchi, C. P., Maestri, M., & Barros, R. S. (2007). Ecophysiology of coffee growth and production. Brazilian Journal of Plant Physiology, 19(4), 485–510.
45. Geromel, C., Ferreira, L. P., Davrieux, F., Guyot, B., Ribeyre, F., dos Santos Scholz, M. B., Pereira, L. F. P., Vaast, P., Pot, D., Leroy, T., & Androcioli, F. A. (2008). Effects of shade on the development and sugar metabolism of coffee (Coffea arabica L.) fruits. Plant Physiology and Biochemistry, 46(5–6), 569–579.
46. Oskoee, M., AghaAlikhani, M., Sefidkon, F., Mokhtassi-Bidgoli, A., & Ayyari, M. (2018). Blessed thistle agronomic and phytochemical response to nitrogen and plant density. Industrial Crops and Products, 122, 566–573.
47. Lambers, H., Chapin III, F. S., & Pons, T. L. (2008). Plant Physiological Ecology. Springer Science & Business Media. University of Western Australia.
48. Ma, S.-C., Li, F.-M., Xu, B.-C., & Huang, Z.-B. (2010). Effect of lowering the root/shoot ratio by pruning roots on water use efficiency and grain yield of winter wheat. Field Crops Research, 115(2), 158–164.
49. Yang, Y., Dou, Y., & An, S. (2017). Environmental driving factors affecting plant biomass in natural grassland in the Loess Plateau, China. Ecological Indicators, 82, 250–259.
50. Bertamini, M., Muthuchelian, K., Rubinigg, M., Zorer, R., Velasco, R., and Nedunchezhian, N (2006). Low-night temperature increased the photoinhibition of photosynthesis in grapevine (Vitis vinifera L. cv. Riesling) leaves. Environ. Exp. Bot., 57(1-2): 25-31.
51. Wittmann, C., Aschan, G., & Pfanz, H. (2001). Leaf and twig photosynthesis of young beech (Fagus sylvatica) and aspen (Populus tremula) trees grown under different light regime. Basic and Applied Ecology, 2(2), 145–154.
52. Bailey, S., Walters, R. G., Jansson, S., & Horton, P. (2001). Acclimation of Arabidopsis thaliana to the light environment: the existence of separate low light and high light responses. Planta, 213(5), 794–801.
53. Jiang, W., Wang, K., Wu, Q., Dong, S., Liu, P., & Zhang, J. (2013). Effects of narrow plant spacing on root distribution and physiological nitrogen use efficiency in summer maize. The Crop Journal, 1(1), 77–83.
54. Labrooy, C. D., Abdullah, T. L., Abdullah, N. A. P., & Stanslas, J. (2016). Optimum shade enhances growth and 5,7-Dimethoxyflavone ac-cumulation in Kaempferia parviflora Wall. ex Baker cultivars. Scientia Horticulturae, 213, 346–353.
55. Omar, N. F. (2018). Growth and phytochemical composition of Andrographis paniculata (Burm.F.) Wall. ex Nees in relation to different light intensities, photoperiod, and pruning. Universiti Putra Malaysia, Serdang, Malaysia.
56. Wang, C., Liu, W., Li, Q., Ma, D., Lu, H., Feng, W., Xie, Y., Zhu, Y., & Guo, T. (2014). Effects of different irrigation and nitrogen regimes on root growth and its correlation with aboveground plant parts in high-yielding wheat under field conditions. Field Crops Research, 165, 138–149.
57. Casey, C. A., Mangan, F. X., Herbert, S. J., Barker, A. V, & Carter, A. K. (2002). The effect of light intensity and nitrogen fertilization on plant growth and leaf quality of ngo gai (Eryngium foetidum L.) in Massachusetts. In XXVI International Horticultural Congress: The Future for Medicinal and Aromatic Plants 629, 215–229.
58. Kumar, R., Sharma, S., & Pathania, V. (2013). Effect of shading and plant density on growth, yield and oil composition of clary sage ( Salvia sclarea L.) in north western Himalaya. Journal of Essential Oil Research, 25(1), 23–32.
59. Urbas, P., & Zobel, K. (2000). Adaptive and inevitable morphological plasticity of three herbaceous species in a multi-species community: field experiment with manipulated nutrients and light. Acta Oecologica, 21(2), 139–147.
60. Ezz, A. L. (2009). Plant growth strategies of Thymus vulgaris L. in response to population density. Industrial Crops and Products, 30(3), 389-394.
61. ZHANG, M. C., SUN, W. X., LIU, Y. Y., LUO, S. G., Jing, Z., Qiong, W. U., ... & Jiang, Y. (2014). Timing of N application affects net primary production of soybean with different planting densities. Journal of Integrative Agriculture, 13(12), 2778-2787.
62. Deng, J., Wang, G., Morris, E. C., Wei, X., Li, D., Chen, B. M., Zhao, B. M., Liu. J., & Wang, Y. (2006). Plant mass–density relationship along a moisture gradient in north‐west China. Journal of Ecology, 94(5), 953–958.
63. Schachtman, D. P., & Goodger, J. Q. D. (2008). Chemical root to shoot signalling under drought. Trends in Plant Science, 13(6), 281–287.
64. Liu, T., Gu, L., Dong, S., Zhang, J., Liu, P., & Zhao, B. (2015). Optimum leaf removal increases canopy apparent photosynthesis, 13C-photosynthate distribution and grain yield of maize crops grown at high density. Field Crops Research, 170, 32–39.
65. Fang, X., Li, Y., Nie, J., Wang, C., Huang, K., Zhang, Y., Zhang, Y., She, H., Liu, X., Ruan, R., &Yuan, X. (2018). Effects of nitrogen fertilizer and planting density on the leaf photosynthetic characteristics, agronomic traits and grain yield in common buckwheat (Fagopyrum esculentum M.). Field Crops Research, 219, 160–168.
66. Derks, H., Mitchell, R. A. C., Mitchell, V. J., & Lawlor, D. W. (1998). Response of sugar beet (Beta vulgaris L.) yield and biochemical composition to elevated CO2 and temperature at two nitrogen applications. Plant, Cell & Environment, 21(8), 829–836.
67. Xie, X., Shan, S., Wang, Y., Cao, F., Chen, J., Huang, M., & Zou, Y. (2019). Dense planting with reducing nitrogen rate increased grain yield and nitrogen use efficiency in two hybrid rice varieties across two light conditions. Field Crops Research, 236, 24–32.
68. Ali, A., Ahmad, A., Khaliq, T., & Akhtar, J. (2012). Planting density and nitrogen rates optimization for growth and yield of sunflower (He-lianthus annuus L.) hybrids. The Journal of Animal and Plant Sciences, 22(4), 1070–1075.
Received: October 9th 2023/ Accepted: January 15th 2024 / Published:15 February 2024
Citation: Ahmed, O.; Yusoff1, M.; Wahab, A. M., Muttaleb, Q. Root-shoot ratio and its relationships with physiological characteristics, growth and biomass yield of Gynura procumbens under different shade levels and plant density. Bionatura Journal 2024; 1 (1) 52. http://dx.doi.org/10.21931/BJ/2024.01.01.52
Additional information Correspondence should be addressed to [email protected]
Peer review information. Bionatura Journal thanks anonymous reviewer(s) for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
All articles published by Bionatura Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Publisher's Note: Bionatura Journal stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2024 by the authors. They were submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
