2024..01.01.62
Files > Conference Series > 2024 > Chimboazo ild pagina nueva
The Role of Quorum Quenching in Medical Application
Ghada A. Mohammad1, Huda Waleed Hadi2*
1University of Mosul / Mosul City / Iraq. [email protected]
2University of Mosul / Mosul City / Iraq. [email protected].
* Correspondence: [email protected] Tel.: 0750216632 Iraq code: +964
Available from. http://dx.doi.org/10.21931/BJ/2024.01.01.62
ABSTRACT
The attempts are continuing in the various fields of life sciences to resolve a big problem, which is the ability of bacteria to cause pathogenicity for humans, animals, and plants, whether by chemical or biological methods and in ways that are hoped to be safe. Among these attempts, the control of the Quorum Sensing (QS) mechanism that occurs naturally in bacteria under certain conditions helps to increase the virulence of bacteria, starting from its ability to adhere and form a biofilm. Then, the tissues are invaded with various enzymes according to the tissue type, increasing antibiotic resistance. Therefore, the idea came to solve these problems through a mechanism opposite to the Quorum Quenching (QQ), which lies in the investigation of substances that can disrupt the QS pathway, whether at the molecular level or the physiological level, as well as benefiting from different organisms (Prokaryotes or Eukaryotes) that live in the same environment and produce substances that inhibit bacterial signaling molecules. Lastly, the discovery of varying novel QQ agents from extreme environmental bacteria will be most interesting in the future.
Keywords: Quorum sensing, quorum quenching, acyl homoserine lactones, medical application.
INTRODUCTION
The population density and bacterial behavior in different environments are regulated by a specific mechanism known as Quorum Sensing (QS). Small molecules responsible for QS are called signaling molecules or Auto-inducers (AIs), sensed by intracellular or membrane-bound receptors in the producer cell or other bacterial cells in the population 1, 2.
There are different types of AIs according to their chemical structure and mechanism of action. The main types of AIs are (i) Acyl Homoserine Lactones (AHLs) that are used by gram harmful bacteria, (ii) Auto-Inducing Peptides (AIPs) in gram-positive bacteria 3. Other references add a third type of AI: furanosyl diester borate (AI-2) 4. Meanwhile, others divide AI-1 into two types: AHLs that carry out intraspecies communications and AI-2 that involve furanosyl borate interspecies communications. 5. Furthermore, studies can provide additional AI types, such as fatty acids (FA), quinolone in Pseudomonas spp., and butrylactone in Streptomyces spp. 1.
Various AI types have the same function as QS molecules when their concentration increases to a certain level. At this concentration level, AIs bind with their receptors (Figure 1). This binding stimulates the regulation of specific genes 6, 3.
AHL molecules may perform other functions as antibacterial agents, mainly when composed of long carbon chains 7, such as N-3-oxo-dodecanoyl-L-homoserine Lactone (OC12-HSL), which are named bacteriocin when they act as antibiotics 8.
Cascades of QS enhance virulence factors such as biofilm production, plasmid transfer, enzymes, and motility. Also, QS gives additional bacterial adaption properties to harsh environmental conditions and activation of bioluminescence in Vibrio fisheri bacteria 9, 6.
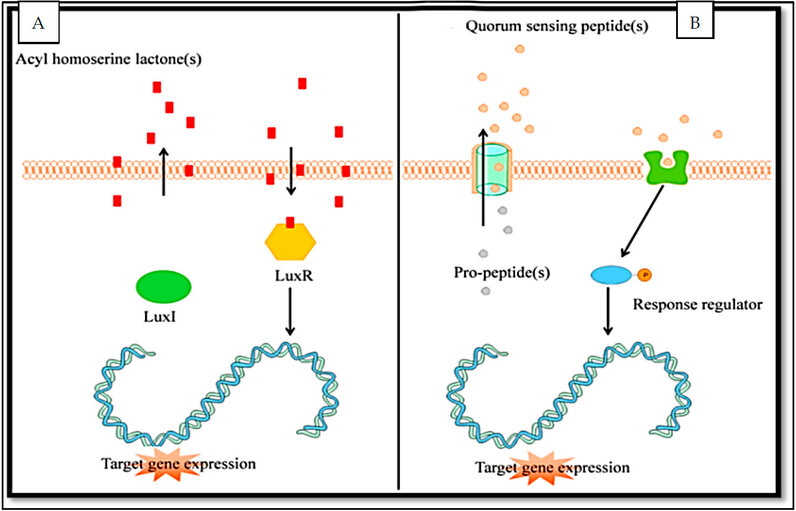
Figure 1: The quorum sensing mechanism. A: in Gram-negative. B: in Gram-positive bacteria 3.
QUORUM QUENCHING (QQ)
The Quorum Quenching (QQ) is a molecular mechanism of processes that interrupt bacterial communication; it is achieved by several modes of action 10- 12. QQ was first discovered in Erwinia carotovora, which produces AHL-degrading enzymes that cause QS blocking 12, 13. QS Pathway consists of multiple steps. Each step can be interfered with by specific QQ molecules, also called QS inhibitors (QSIs). Biological molecules and other physical factors such as pH and temperature can act as QSIs. 6.
The competitive inhibitors for AI molecules can be biosynthesized from microorganisms such as bacteria or eukaryotic organisms like algae, marine animals, plants, and fungi. These inhibitors block AIs-receptors binding, leading to QS disrupting 14. Extracellular QQ enzymes can degrade or modify AIs. These enzymes produce bacteria with competitive advantages to get nutrients in their environments 15, 16.
There is no pathogenic commensal flora present in healthy skin. When the skin is exposed to wounds, lesions, and burns, this flora colonizes damaged skin, multiplying and forming biofilm. Signaling molecules AIs produce a biofilm that enables bacteria to become more virulent and resistant to antibiotics, phage therapy, and antibody goals. However, the QQ molecules that are used against biofilm bacteria remain not harmful and without defenses (Figure 2). Therefore, QQ helps cure unhealthy skin and helps it stay in a noninfectious state 17.
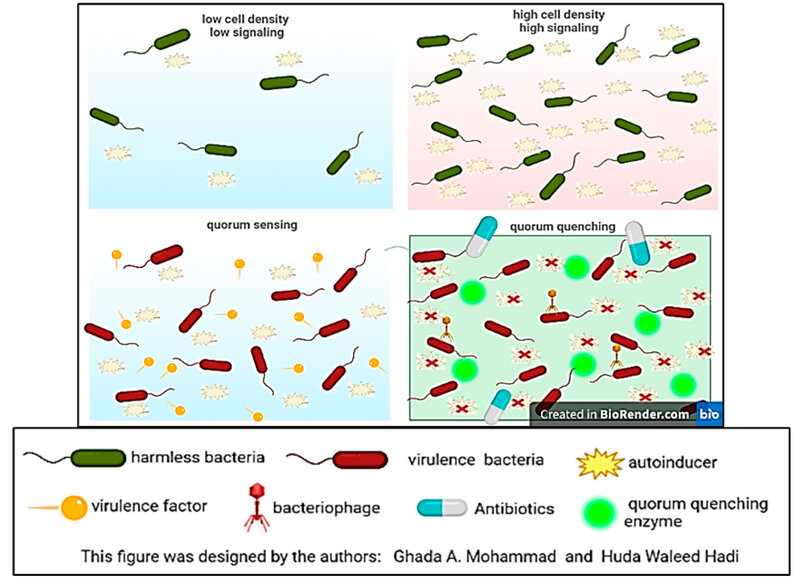
Figure 2: Quorum sensing vs. quorum quenching in this review.
FIRST: NATURAL COMPOUNDS
Natural compounds have important antibacterial features and anti-virulence characteristics. QQ molecules from natural compounds, either eukaryotic or prokaryotic sources, are efficient and safe. They are readily biodegradable and can interrupt bacterial infection in a very active manner 18, 19.
A- Bacterial QSIs
Bacterial QQ molecules are isolated from different bacterial taxa such as Firmicutes, Actinobacteria, Proteobacteria, Bacteroides, and Cyanobacteria. These bacterial QQs are very active against Pseudomonas aeroginosa biofilm producers and also have an essential role in biofilm removal in the wastewater treatment plant. The bacteria produce three types of enzymes: AHL lactonase, AHL acylase, and AHL oxide reductase 13, 11. Other references add a fourth class to these enzymes to become four classes by separating oxidase enzymes such as cytochrome from reductase enzymes 20, 1.
AHL-Lactonase Enzymes
This type of enzyme belongs to the Metallo-β-Lactamase (MBL) family 11. Some references further divided the lactonase enzymes in addition to the previous class according to their protein folding and active motif, such as the Phosphodiesterase- Lactonase (PLLs), the α/β hydrolase fold lactonase, and the paraoxonase. Classes of lactonase have been observed evolutionary convergence with low specificity to the substrate level except PLLs that show high specificity to the long chains AHL 16, 21. Lactonase enzymes can degrade or modify the lactone ring (Figure 3) in different molecules of AHL, therefore blocking swarming motility in P. aeroginosa by lactonase produced by Pectobacterium carotovora22. Hence, they are efficient in interfering with QS. AHL-lactonase enzymes have been isolated from various prokaryotic, archaea, and eukaryotic organisms. They have been assessed in their controlling of different diseases. Therefore, increasing their effectiveness activity by engineering studies leads to the rise of their catalytic activity and stability 23.
Lactonase enzymes do not interfere with QS only; they can interfere with other biological processes, such as the bacterium Agrobacterium tumefacient, which is controlled by Ti plasmid 24, 1.
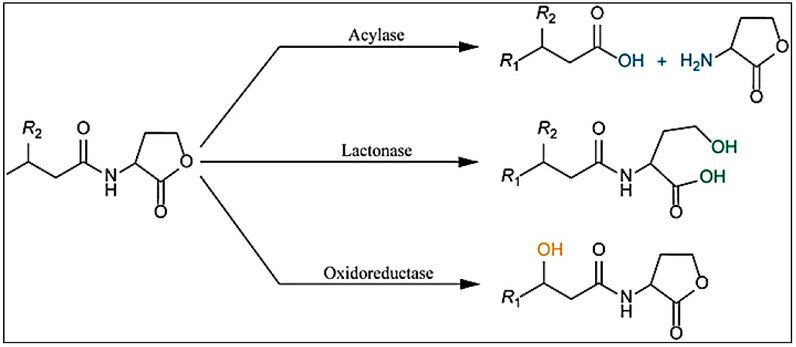
Figure 3: Prokaryotic Enzymes Action on AHL molecoule 25.
AHL-Acylase Enzymes
Acylase enzymes separate acyl chains from lactone rings (Figure 3). Amidase enzymes (or acylase) are considered from this type of enzyme. P. aeroginosa can degrade the AHL molecules by its amidase enzymes 1. It has been proved that treatment of wastewater biofilms by AHLs degrading enzymes (Aculeacin A Acylase (AuAAC) which is obtained from Actinoplanes utahensis) can prevent the four systems of QS in P. aeroginosa 13.
AHL-acylase enzymes belong to the family of N-terminal nucleophilic hydrolases (Ntn- hydrolases). PvdQ is an example of these enzymes that can split an acyl chain with more than 10 carbon atoms 16.
AHL-Oxidoreductase Enzymes
These enzymes can modify the AHL molecules without degrading them (Figure 3). They can reduce or oxidize AHL molecules 26. Oxidoreductase enzymes spread in the bacteria that used AHL molecules as nitrogen or carbon sources 11. also include oxidoreductase and esterase enzymes 26.
B- Eukaryotic QSI
Varieties of QQ eukaryotic molecules have been isolated from different taxa of the eukaryotic cells. These molecules are very active as QQ; therefore, they have essential roles in medical fields20,27. Eukaryotic enzymes have been extracted and purified from different animals, which can disrupt QS signals, such as the acylase 1 enzyme of porcine kidney 28. paraxonases 1, 2, 3, and QQ enzymes are found in epithelial cells and mammalian sera 29.
However, the plant extracts represent valuable and safe compounds for disrupting the bacterial QS (Table 1). Because of the ability of these compounds to disrupt bacterial infection without causing antibacterial resistance, QQ phytocompounds are very important in fighting bacterial virulence and antibiotic resistance, especially in P. aeruginosa 19.
Isolated substances from fungi also have a role in the QS blocking, such as ethyl acetate extracts from Plectosphaerella cucumerina fungus against P. aeroginosa PAO1 strain; the treatments by the fungal compounds lead to a high potential in blocking the biofilm formation without killing the producing bacteria30.
The marine red algae Halemenia durvilleican produces metabolites that disrupt the AHL in gram-negative bacteria such as Klebsiella pneumonia and P. aeroginosa 31. Complex reactions can occur in the relationships between algae and QS-associated bacteria, which may affect their ecology system 32.
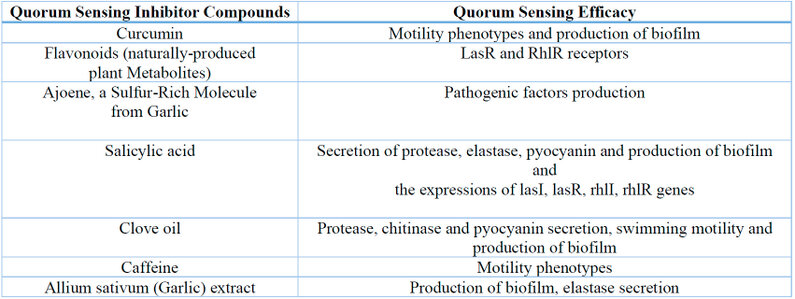
Table 1: The Herbal Extracts as Quorum Sensing Inhibitors 11
SECOND: SYNTHETIC QSI
The use of nanoparticles (NPs) and engineering nanoparticles as antimicrobial agents gained importance in previous years because of the increased bacterial resistance to ward antimicrobial agents. NPs have little or no toxicity and have pharmaceutical properties as antibiotics 33, 34, 35. Other studies suggested that NPs can be used as anti-QS signals. Because of its ability to penetrate the biofilm, genes cascade and stop cell-to-cell signaling, which leads to the cessation of biofilm formation 33, 36.
Cationic polysaccharides chitosan NPs have many clinical properties, but the important one is anti-QS signals. It has a positive charge that reacts with the negative charge of the lipopolysaccharides in the P. aeroginosa cell wall. This reaction causes membrane permeability changes, leading to bacterial killing; furthermore, the NPs can affect the virulence factors such as pyocyanin and protease gene expression of biofilm formation 37.
The zinc oxide NPs can combat P. aeroginosa signals such as rhamnolipid, pyocyanin, pyoverdin, hemolysin, elastase, and protease in addition to the inhibition of QS genes, which leads to a decrease in the pathogenicity and virulence of P. aeroginosa in vivo 38.
Quorum Quenchers Application
The QQ has an essential role in the medical field, health care, aquaculture, agriculture purposes, phytochemicals, antibiotics, and water engineering 39.
Quorum Quenching in Medical Application
The QQ controls various virulence factors such as sporulation, biofilm formation, enzyme production, motility, pigment production, flagella, and pili40. Also, biofilm formation is the main problem that makes most diseases, such as oral cavities and cystic fibrosis, hardly curing41.
There are several mechanisms in the medical application of QQ, such as:
1- Using synthetic analogs of AHL molecules, which disrupt the binding of AHLs with their receptors, can also alter the chemical function of the acyl chain tail or the head of AHL molecules 42.
2- Using engineering QQ enzymes such as lactonase and acylase isolated from various organisms may become more efficient in the degradation of AHL molecules 23.
3- Down-regulation of the bacterial virulence genes by Qs inhibitors using trans-cinnamaldehyde and salicylic acid in QS inhibition of P. aeroginosa PAO1 strain 43.
4- Blocking the signaling cascades 44.
5- Inhibition of the signal molecules' biosynthesis 45.
In the field of Medical Devices
Most Hospital Acquired Infections (HAIs) are associated with using various contaminated medical devices 46. The emergence of QQ molecules in synthesizing various medical devices is a very useful method to avoid HAIs (Table 2). The recent generation of catheters, trauma, dressing, aerosols, contact lenses, and implantable devices are involved in QQ molecules 17. For example, device surfaces coated with pro and anti-QS peptides show high antibacterial potential against Staphylococcus aureus strains 47.
Medical devices containing QQ require further testing with more pathogenic and virulent bacterial strains. Therefore, researchers seek to use highly resistant QQ enzymes or compounds isolated from microorganisms of extreme environments, such as Solfolobus solfataricus 17.
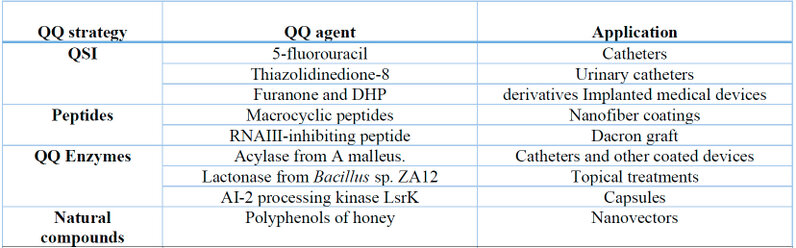
Table 2: Quorum quenching-based medical devices 17.
QQ-Based Antibacterial Treatments in Medicine
Conventional antibiotics cannot penetrate the extracellular matrix of bacterial biofilm, which leads to the loss of 80% of their efficiency. The infections caused by biofilm production in oral cavities and cystic fibrosis occur directly via this biofilm, and most infections associated with HAIs result from biofilm contamination of medical devices48 41.
Antibody-based QQ efforts
The blocking of bacterial cell communication via the use of monoclonal antibodies (mAbs) is a beautiful strategy to prevent infections. These mAbs can also detect homoserine lactones (HSLs), which control QS system-dependent LuxI/LuxR in P. aeroginosa. The groups of mice treated with the QQ mAbs are found to be protected and cured from pneumonia caused by P. aeroginosa compared with control groups 49.
The mAbs-based QQ have acquired importance in recent years because of their high specificity and little toxicity; they also can degrade the signaling molecules by inhibiting the pyocyanin production in P. aeroginosa50.
Antibiotics as QSI
Antibiotics cannot inhibit the growth, metabolic activities, or cell wall synthesis of bacteria only. Still, they also interfere with the QS pathways as macrolides and β -lactam antibiotics, inhibiting the several appearances of QS51.
Azithromycin, imipenem, cefepime, tazobactam, and piperacillin were examined as antivirulence factors of wild and mutant P. aeroginosa strains. Virulence factors include pyocyanin, biofilm, hemolysin, protease, and DNAse, all of which depend on QS signals. Antibiotics in their sub-inhibitory concentration (SIC) can interfere with QS better than the highest concentrations and do not cause the appearance of antibacterial resistance and side effects52.
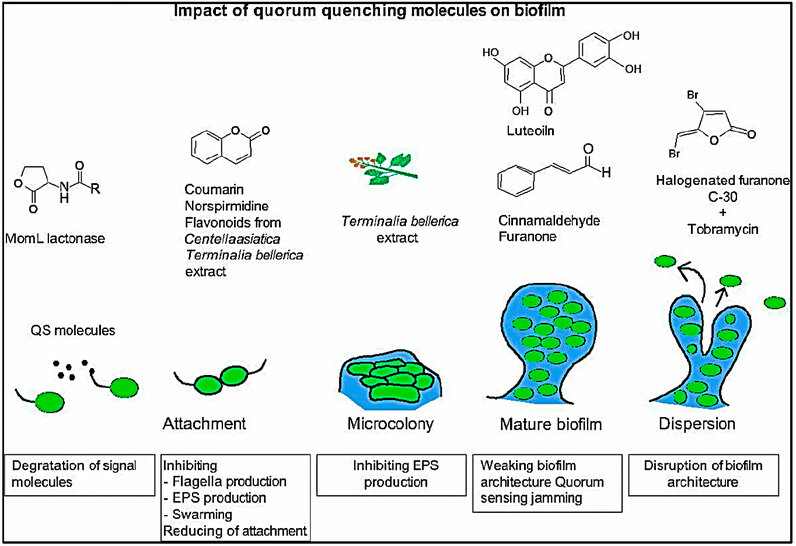
Figure 4: The impact of quorum quenching molecules on biofilm formation 6.
Similar results with P. aeroginosa and Acinetobacter baumannii, which are considered from MDR bacteria, were obtained by the study of Saleem and co-workers, who mentioned that the Erythromycin, chloroquine, and propranolol antibiotics were able to inhibit (in SIC) virulence based on QS such as biofilm, enzymes, resistance to oxidative stress, swarming and twitching 53.
CONCLUSIONS
As bacterial response mechanisms are regulated by what is known as Quorum Sensing QS, other mechanisms meet it and work to stop it, and they are called Quorum Quenching QQ. Both QS and QQ mechanisms are diverse and vary with a wide range of chemical compositions. So, scientists compete to reach the most efficient means of QQ by various means to eliminate the bacterial pathogenicity and virulence factors, especially in resistant pathogenic bacteria. Nowadays, the QQ methods represent a global goal to eliminate antibiotic resistance mechanisms that have recently spread comprehensively. Therefore, this article highlights the most important and latest applications based on QQ, especially medical equipment and devices in direct contact with the patient's life, to eliminate hospital nosocomial infections and the widespread bacterial resistance to the latest choice of antibiotics.
Conflicts of Interest: "The authors declare no conflict of interest." "The funders had no role in the study's design; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results."
REFERENCES
1. Grandclément, C., Tannières, M., Moréra, S., Dessaux, Y., Faure, D. Quorum quenching: role in nature and applied developments. FEMS microbiology reviews 2016, 40:86-116.
2. Xiao, Y., Zou, H., Li, J., Song, T., Lv, W., Wang, W., ... Tao, S. Impact of quorum sensing signaling molecules in gram-negative bacteria on host cells: current understanding and future perspectives. Gut Microbes 2022; 14: 2039048.
3. Verbeke, F., De Craemer, S., Debunne, N., Janssens, Y., Wynendaele, E., Van de Wiele, C., De Spiegeleer, B. Peptides as quorum sensing molecules: measurement techniques and obtained levels in vitro and in vivo. Frontiers in neuroscience 2017, 11, 183.
4. Huang, J., Shi, Y., Zeng, G., Gu, Y., Chen, G., Shi, L., ... Zhou, J. Acyl-homoserine lactone-based quorum sensing and quorum quenching hold promise to determine the performance of biological wastewater treatments: an overview. Chemosphere 2016, 157, 137-151.
5. Hmelo, L. R. Quorum sensing in marine microbial environments. Annual review of marine science 2017, 9, 257-281.
6. Paluch, E., Rewak-Soroczyńska, J., Jędrusik, I., Mazurkiewicz, E., Jermakow, K. Prevention of biofilm formation by quorum quenching. Applied microbiology and biotechnology 2020, 104, 1871-1881.
7. Daniels, R., Reynaert S.,HoekstraH, et al. Quorum signal molecules as biosurfactants affect swarming in Rhizobium etli. P NatlAcad Sci USA 2006,103:14965–70.
8. Lowery, C.A., Park, J., Gloeckner, C., et al. We are defining the mode of action of tetramic acid antibacterials derived from Pseudomonas aeruginosa quorum sensing signals. J Am Chem Soc 2009;131:14473–9.
9. Nazzaro, F., Fratianni, F., Coppola, R. Quorum sensing and phytochemicals. International journal of molecular sciences 2013, 14(6), 12607-12619.
10. Natrah, F. M. I., Kenmegne, M. M., Wiyoto, W., Sorgeloos, P., Bossier, P., Defoirdt, T. Effects of micro-algae commonly used in aquaculture on acyl-homoserine lactone quorum sensing. Aquaculture 2011, 317(1-4), 53-57.
11. Hemmati, F., Salehi, R., Ghotaslou, R., Kafil, H. S., Hasani, A., Gholizadeh, P., Nouri, R. Rezaee, M. A. Quorum quenching: A potential target for antipseudomonal therapy. Infection and Drug Resistance 2020, 13, 2989.
12. Dong, Y. H., Xu, J. L., Li, X. Z., Zhang, L. H. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proceedings of the National Academy of Sciences 2000, 97(7), 3526-3531.
13. De Celis, M., Serrano-Aguirre, L., Belda, I., Liébana-García, R., Arroyo, M., Marquina, D., ... Santos, A. Acylase enzymes disrupting quorum sensing alter the transcriptome and phenotype of Pseudomonas aeruginosa, and the composition of bacterial biofilms from wastewater treatment plants—science of the Total Environment 2021, 799, 149401.
14. Surpeta, B., Grulich, M., Palyzová, A., Marešová, H., Brezovsky, J. Common Dynamic Determinants Govern Quorum Quenching Activity in N-Terminal Serine Hydrolases. ACS Catalysis 2022, 12, 6359-6374.
15. Sikdar, R., Elias, M. Quorum quenching enzymes and their effects on virulence, biofilm, and microbiomes: a review of recent advances. Expert review of anti-infective therapy 2020, 18(12), 1221-1233.
16. Rehman, Z. U., Leiknes, T. Quorum-quenching bacteria isolated from Red Sea sediments reduce biofilm formation by Pseudomonas aeruginosa—frontiers in microbiology 2018, 9, 1354.
17. Rémy, B., Mion, S., Plener, L., Elias, M., Chabrière, E., Daudé, D. Interference in bacterial quorum sensing: a biopharmaceutical perspective. Frontiers in pharmacology 2018, 9, 203.
18. Asfour, HZ. Anti-quorum sensing natural compounds. Journal of microscopy and ultrastructure 2018, 6(1), 1.
19. Moradi, F., Hadi, N. Quorum-quenching activity of some Iranian medicinal plants. New Microbes and New Infections 2021, 42, 100882.
20. Chowdhary, P. K., Keshavan, N., Nguyen, H. Q., Peterson, J. A., González, J. E., Haines, D. C. Bacillus megaterium CYP102A1 oxidation of acyl homoserine lactones and acyl homoserines. Biochemistry 2007, 46(50), 14429-14437.
21. Hiblot, J., Bzdrenga, J., Champion, C., Chabriere, E., Elias, M. Crystal structure of VmoLac, a tentative quorum quenching lactonase from the extremophilic crenarchaeon Vulcanisaeta moutnovskia. Scientific Reports 2015, 5(1), 1-11.
22. Dong, W., Zhu, J., Guo, X., Kong, D., Zhang, Q., Zhou, Y., Liu, X., Zhao, S. Ruan, Z. Characterization of AiiK, an AHL lactonase, from Kurthia huakui LAM0618T and its application in quorum quenching on Pseudomonas aeruginosa PAO1. Scientific reports 2018, 8(1), 1-11.
23. Billot, R., Plener, L., Jacquet, P., Elias, M., Chabrière, E., Daudé, D. Engineering acyl-homoserine lactone-interfering enzymes toward bacterial control. Journal of Biological Chemistry 2020, 295(37), 12993-13007.
24. Haudecoeur, E., Tannières, M., Cirou, A., Raffoux, A., Dessaux, Y., Faure, D. Different regulation and roles of lactonases AiiB and AttM in Agrobacterium tumefaciens C58. Molecular plant-microbe interactions 2009, 22(5), 529-537.
25. Chen, F., Gao, Y., Chen, X, Yu, Z., Li, X. Quorum Quenching Enzymes and Their Application in Degrading Signal Molecules to Block Quorum Sensing-Dependent Infection. International Journal of Molecular Sciences 2013, 14:17477 - 17500.
26. Utari, P. D., Vogel, J., Quax, W. J. Deciphering physiological functions of AHL quorum quenching acylases. Frontiers in microbiology 2017, 8, 1123.
27. AL-Sabagh, F. S. H., Ghaima, K. K., Sh.AL-Dabbagh, A. H. The antibacterial activity of LL-37 peptide against multidrug-resistant Pseudomonas aeruginosa isolated from burn infections. Revis Bionatura 2023, 8 (1), 69.
28. Dong, Y. H., Zhang, L. H. Quorum sensing and quorum-quenching enzymes. Journal of Microbiology 2005, 43(spc1), 101-109.
29. LaSarre, B., Federle, M. J. Exploiting quorum sensing to confuse bacterial pathogens. Microbiology and molecular biology reviews 2013, 77(1), 73-111.
30. Zhou, J.W., Chen, T.T., Tan, X.J., Sheng, J.Y., Jia, A.Q. Can the quorum sensing inhibitor resveratrol function as an aminoglycoside antibiotic accelerant against Pseudomonas aeruginosa?. International journal of antimicrobial agents 2018, 52: 35-41.
31. Boominathan, R., Devanesan, S., AlSalhi, M.S., Balasubramanian, A., Alkhalid, I.Z., Paul, P., Singh, AR. Quorum quenching action of marine red alga Halemenia durvillei on biofilm forming Gram negative bacterial isolates from contact lens. Algal Research 2022, 64, 102693.
32. Zhang, Y., Zheng, L., Wang, S., Zhao, Y., Xu, X., Han, B., Hu, T. Quorum sensing bacteria in the phycosphere of hab microalgae and their ecological functions related to cross-kingdom interactions. International Journal of Environmental Research and Public Health 2022, 19(1), 163.
33. Pelgrift, R.Y., Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Advanced drug delivery reviews 2013, 65, 1803-1815.
34. Selah, M. T., Mohammad, G. A. Ability of three species of Enterobacter bacteria to synthesize iron nanoparticles and detection of the efficacy to inhibitory effect on other pathogenic bacteria. Biochemical and Cellular Archives 2021, 21, 2085-2090.
35. Ahmed, A.M., Al Marjani, M.F., Rheimah, A.M. Antibacterial and anti-biofilm action of cobalt oxide nanoparticles beside persister Pseudomonas aeruginosa isolates. Revis Bionatura 2023,8 (2), 78.
36. DAS, Bhaskar; PATRA, Sanjukta. Antimicrobials: Meeting the challenges of antibiotic resistance through nanotechnology. En Nanostructures for antimicrobial therapy. Elsevier, 2017. p. 1-22.
37. Qais, F.A., Khan, M.S., Ahmad, I. Nanoparticles as quorum sensing inhibitor: Prospects and limitations. Springer, Singapore. In Biotechnological applications of quorum sensing inhibitors 2018. 227-244.
38. Ma, Z., Garrido-Maestu, A., Jeong, K. C. Application, mode of action, and in vivo activity of chitosan and its micro-and nanoparticles as antimicrobial agents: A review. Carbohydrate polymers 2017, 176, 257-265.
39. Saleh, M. M., Refa't A, S., Latif, H. K. A., Abbas, H. A., Askoura, M. Zinc oxide nanoparticles inhibits quorum sensing and virulence in Pseudomonas aeruginosa. African health sciences 2019, 19(2), 2043-2055.
40. Maddela, N. R., García Cruzatty, L. C., Leal-Alvarado, D. A., Olaya, J. C., Chakraborty, S., Mukherjee, A. Quorum Quenching for Sustainable Environment: Biology, Mechanisms, and Applications. Springer, Singapore.In Microbial Technology for Health and Environment 2020 (pp. 73-112).
41. Krzyżek, P. Challenges and limitations of anti-quorum sensing therapies. Frontiers in microbiology 2019, 10, 2473.
42. Pawlaczyk-Kamieńska, T., Borysewicz-Lewicka, M., Batura-Gabryel, H., Cofta, S. Oral Care Recommendation for Cystic Fibrosis Patients–Recommendation for Dentists. Journal of Clinical Medicine 2022, 11(10), 2756.
43. Palmer, A. G., Senechal, A. C., Haire, T. C., Mehta, N. P., Valiquette, S. D., Blackwell, H. E. Selection of appropriate autoinducer analogues for the modulation of quorum sensing at the host–bacterium interface. ACS chemical biology 2018, 13(11), 3115-3122.
44. Ahmed, S.A., Rudden, M., Smyth, T. J., Dooley, J. S., Marchant, R., Banat, I. M. Natural quorum sensing inhibitors effectively downregulate gene expression of Pseudomonas aeruginosa virulence factors. Applied microbiology and biotechnology 2019, 103(8), 3521-3535.
45. Oh, M. H., Choi, C. H. Role of LuxIR homologue AnoIR in Acinetobacter nosocomialis and the effect of virstatin on the expression of anoR gene. Journal of Microbiology and Biotechnology 2015, 25(8), 1390-1400.
46. Lade, H., Paul, D., Kweon, J. H. Quorum quenching mediated approaches for control of membrane biofouling. International journal of biological sciences 2014, 10(5), 550.
47. Neoh, K. G., Li, M., Kang, E. T., Chiong, E., Tambyah, P. A. Surface modification strategies for combating catheter-related complications: recent advances and challenges. Journal of Materials Chemistry B 2017, 5(11), 2045-2067.
48. Kim, M. K., Zhao, A., Wang, A., Brown, Z. Z., Muir, T. W., Stone, H. A., Bassler, B. L. Surface-attached molecules control Staphylococcus aureus quorum sensing and biofilm development. Nature Microbiology 2017, 2(8), 1-12.
49. Lebeaux, D., Chauhan, A., Rendueles, O., Beloin, C. From in vitro to in vivo models of bacterial biofilm-related infections. Pathogens 2013, 2(2), 288-356.
50. Palliyil, S. Quorum Quenching Monoclonal Antibodies for the Detection and Treatment of Gram-Negative Bacterial Infections. Trends in Quorum Sensing and Quorum Quenching 2020, 285-290.
51. Jiang, Q., Chen, J., Yang, C., Yin, Y., Yao, K. Quorum sensing: a prospective therapeutic target for bacterial diseases. BioMed Research International 2019, 2019.
52. El-Mowafy, SA., Abd El Galil, KH., Habib, ES. E., Shaaban, MI. Quorum sensing inhibitory activity of sub-inhibitory concentrations of β-lactams. African Health Sciences 2017, 17(1), 199-207.
53. Aleanizy, FS., Alqahtani, FY., Eltayb, EK., Alrumikan, N., Almebki, R., Alhossan, A., ... AlQahtani, H. Evaluating the effect of antibiotics sub-inhibitory dose on Pseudomonas aeruginosa quorum sensing dependent virulence and its phenotypes. Saudi Journal of Biological Sciences 2021, 28(1), 550-559.
54. Seleem, N. M., Abd El Latif, H. K., Shaldam, M. A., & El-Ganiny, A. Drugs with new lease of life as quorum sensing inhibitors: For combating MDR Acinetobacter baumannii infections. European Journal of Clinical Microbiology & Infectious Diseases 2020, 39(9), 1687-1702.
Received: October 9th 2023/ Accepted: January 15th 2024 / Published:15 February 2024
Citation: Mohammad G. A., Hadi H. W. The Role of Quorum Quenching in Medical Application. on the Mortality of Two Nematodes in a Laboratory Setting. Revis Bionatura 2024; 1 (1) 62. http://dx.doi.org/10.21931/BJ/2024.01.01.62
Additional information Correspondence should be addressed to [email protected]
Peer review information. Bionatura thanks anonymous reviewer(s) for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
All articles published by Bionatura Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Publisher's Note: Bionatura stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2024 by the authors. They were submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
