2024..01.01.35
Files > Conference Series > 2024 > Chimboazo ild pagina nueva
Rosmarinus Officinalis: Phytochemical analysis and biological activities
Dalila bencheikh1,2*, Khawla laichi1,a, Chemseddine herizi1,b, Mebarka ahmed Azi1,c, Seddik khennouf 2,a, Saliha dahamna 2,b
1*Department of Biochemistry and Microbiology, Faculty of Sciences, University of M'sila, PO Box166 Ichebilia,28000 Msila, Algeria.
2Laboratory of phytotherapy applied to chronic diseases. Department of Animal Biology and Physiology
1, a Department of Biochemistry and Microbiology, Faculty of Sciences, University of M'sila, PO Box166 Ichebilia,28000 Msila, Algeria.
1, b Department of Biochemistry and Microbiology, Faculty of Sciences, University of M'sila, PO Box166 Ichebilia,28000 Msila, Algeria.,
1, c Department of biochemistry and microbiology, Faculty of Sciences, University of M’sila, PO Box166 Ichebilia,28000 Msila, Algeria.,
2, a Laboratory of phytotherapy applied to chronic diseases. Department of Animal Biology and Physiology,
2Laboratory of phytotherapy applied to chronic diseases. Department of Animal Biology and Physiology,
* Correspondence: [email protected]
Available from. http://dx.doi.org/10.21931/BJ/2024.01.01.35
ABSTRACT
Rosemary (Rosmarinus officinalis), a very abundant species in Algeria, is a medicinal plant belonging to the Lamiaceae family, used for its various therapeutic effects. The present study was conducted to determine the bioactive compounds and biological activities (antioxidant and antibacterial activities) of the aqueous extract of the plant (EQRO). The sensitivity of the tested bacterial strains varies according to dilutions and bacterial nature (Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli), which was determined using the agar diffusion method. Meanwhile, the in vitro antioxidant activity was assessed using DPPH radical scavenging. EQRO showed high levels of polyphenols and flavonoid contents (455.10 µg EAG/mg extract; 7.33 µg EAQ / mg extract, respectively) with a yield of 14.47%. In addition, the plant extract revealed a significant antioxidant activity as evidenced by the DPPH (IC50=0.128 mg/ml), which is close to that obtained by BHT. Results showed a remarked antimicrobial effect against gram-positive bacteria (Staphylococcus aureus). At the same time, there was no significant effect on gram-negative bacteria (Pseudomonas aeruginosa and Escherichia coli), which explains the difference in susceptibility of the tested bacterial strains. Rosmarinus officinalis is suggested as an effective therapeutic medicinal plant because of its antioxidant and antibacterial properties.
Keywords: Antibacterial activity, Antioxidant activity, Aqueous extract, bioactive compounds, Rosmarinus officinalis.
INTRODUCTION
Oxidative stress is an imbalance between radical species oxygen (ROS) production and cellular antioxidant capacities1. ROS has long been considered to be a toxic by-product of normal oxygen metabolism and is involved in many pathologies. The effects of free radicals in biology are now well documented; not only have living organisms adapted and coexisting in the presence of ROS, but they have also developed mechanisms to build defenses against them 2.
Bacteria are responsible for several diseases. Their resistance to antibiotics is becoming more pronounced 3. The situation is even more worrying because of the appearance of strains of antibiotic-resistant microorganisms. It is necessary to seek another approach to reduce or eliminate the effects without using synthetic products so that solutions can be found using plant-based bioactive molecules 4.
According to the WHO, 14 to 28% of plants worldwide are listed as having medicinal use 5. Surveys carried out at the beginning of the 21st century reveal that 3 to 5% of patients in Western countries, 80% of rural populations in developing countries and 85% of populations of south Sahara use medicinal plants as the primary treatment 6. Medicinal plants are valuably used in traditional medicine to treat diseases 7; 8
Algeria is very rich in plants, which grow spontaneously 9. Rosmarinus officinalis (family of Lamiaceae), also known as rosemary (in Arabic, 'IKLIL ELDJABEL'), is a medicinal plant native to the Mediterranean region. The dried flower heads, leaves, and an essential oil are used in herbal therapy10. Traditional healers often use this plant effectively in treating various infectious diseases 11. Rosemary contains several active agents responsible for different activities: carnosol, romano, carnosic acid, methyl carbonate, and some flavonoids such as cirsimaritin and genkwanin 12. So, it is a natural antioxidant used as one of the spices with the highest antioxidant activity due to their components 13.
To the best of our knowledge, there are few studies regarding the biological effects of Rosmarinus officinalis. Thus, in this paper, we investigate the potential of Rosmarinus officinalis aqueous extract (EQRO) to enhance the antioxidant activity and antibacterial effect on three strains of bacteria (Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa) and to estimate as well as the amount of phenolics acids.
MATERIALS AND METHODS
Plant Material
The aerial part of Rosmarinus officinalis was collected in June 2021 in the region of Tessala Lemtai, in the extreme north of the wilaya of Mila in eastern Algeria. The town is characterized by a mountainous agricultural character, cold in winter and moderately hot in summer, especially in the community's north. The harvested parts are freed of impurities, dried away from the sun, and placed in a dry, ventilated, shaded place.
Microorganisms
The microbial strains that were the subject of this study were provided by the Microbiology laboratory at the University of M'sila. Algeria. They are maintained by subculturing on nutrient agar favorable to their growth for 24 h at 37 °C. One Gram-positive bacteria, Staphylococcus aureus ATCC 22923, and two Gram-negative bacteria, Escherichia coli ATCC 22922 and Pseudomonas aeruginasa ATCC 27853, were tested.
Preparation of the extract
The aqueous extract of the aerial part of Rosmarinus officinalis is obtained from the decoction method. This was prepared according to 14. 10g of the plant extract is put in 250 ml of distilled water until boiling (100°C) for 15 to 20 minutes, then cooled before being filtered. Then, the extract was stored at -20 °C until use. The yield in dry extract expressed as a percentage is calculated according to the following formula:
Yield% = 𝑴 𝒆𝒙𝒕𝒓ac𝒕 /𝑴𝟎 sample × 𝟏𝟎𝟎
Yield%: percentage yield,
𝐌: dry extract weight (g)
𝐌𝟎: powdered sample weight (g)
Determination of total polyphenols
The total polyphenols content in aqueous extract was determined using the method of 15 with slight modifications. Briefly, a volume of 0.1ml of the extract was added, and their volume made up to 4.5ml of distilled water to give a mixture of 4.6ml and then mixed with 0.1ml of Folin-Ciocalteu reagent (diluted 3 times by distilled water) and 0.3ml of a 2% sodium carbonate solution. After incubation for 2h, the absorbance is read at 760nm. Phenols were expressed as gallic acid equivalents (µg gallic acid/mg dried extract).
Determination of total flavonoid contents
The total flavonoid content in aqueous extract was determined using the method of 16. In brief, 0.25ml of extract with 1.25ml of distilled water was mixed with 0.75ml of NaNO2 solution (5%). After 6 minutes, 150ml of AlCl3 solution (10%) was added. A volume of 0.5 ml of M NaOH was added, and the mixture was made up to 2.5 ml with distilled water. The absorbance is read immediately at 510 nm. Results were expressed as equivalent quercetin (mg quercetin /g dried extract).
Quantification of tannins
The tannin content was estimated using the method of 17. A volume of plant extract was diluted to obtain a concentration of total polyphenols of approximately 500 µg/ml and mixed with an equal volume of hemolyzed sheep blood (absorbance equal to 1.6). After 10 minutes, this solution was centrifuged for 20 minutes, and the absorbance of the supernatant was measured at 576nm. The precipitation efficiency of the tested solutions is expressed in µg of tannic acid equivalent/g of extract.
Antioxidant Activity Assays
DPPH radical scavenging assay
DPPH reactions have often been used to estimate the antiradical activity of the natural products 18 because of the ability of the extract to donate an H atom to free radical by the decrease in its absorbance at 517nm.
According to Chevallier 19, 50µL of different extract concentrations was added to 5ml of the DPPH solution (0,004%). After 30 minutes of incubation, the absorbance is read at 517 nm. The following formula (1) was used to calculate the percentage of free radical DPPH inhibition (I%):
Inhibition % = (A control - A test) х 100 / ABS control1.
With: A Control: is the absorbance of the blank solution; A test is the absorbance of the sample compound.
Antibacterial activity
Conserved bacterial strains were seeded into test tubes containing nutrient broth and then incubated at 37°C for 24 hours to stimulate their development. After bacterial growth, these strains were subcultured on an agar nutrient poured into a Petri dish and then incubated at 37°C for 24 hours. Its opacity must be equivalent to 0.5 Mc Ferland, which corresponds to 108 CFU/ml (Colony Forming Units), then diluted to obtain an inoculum of 106 CFU/ml (Neggaz, 2010)
Antibacterial activity was evaluated by the agar diffusion method known as the disc diffusion method 20. The discs of 9mm in diameter, impregnated with 30µL of the 3 concentrations of the aqueous extract 200, 400 and 600 mg/ml of the EQRO and a disk containing DMSO or Distilled water as a negative control placed in the center of each plate. The medium poured into Petri dishes is inoculated by swabbing from a 106 CFU/ml bacterial suspension. This operation is repeated 3 times. The plates were incubated at 37 °C/24 h. The microbial growth is assessed by measuring the diameters of the zone of inhibition (mm) around the disks.
· Not sensitive (-) or resistant: diameter< 8mm
· Sensitive (+): diameter between 9 to 14 mm
· Very sensitive (++): diameter between 15 to 19 mm
· Extremely sensitive (+++): diameter· > 20 mm 21.
Four synthetic Antibiotics, namely Streptomycin (S: 10µg/disk), Gentamicin (GEN: 10µg/disk), Colistin sulfate (C.S.: 10µg/disk), Cefazolin (CZL: 30µg/disk) were used by the agar diffusion method to detect the sensitivity of the bacterial strains.
Statistic study
All experiments were performed in triplicate. For each test or method, the averages and standard deviations of the tests and the graphic representations were carried out using Excel 2007 software.
RESULTS
Phytochemical analysis
The extraction yield of the aqueous extract of Rosmarinus officinalis by the decoction method was estimated to be 14.49%
As indicated in Table 1, the EQRO revealed high values of total polyphenols content (455.10 ± 0.07 µg EAG/mg of extract) since the high tannins content (135.91±0.020 μg TAE/mg) was noticed in PC AQE. Whereas the total phenolic content in terms of mg GAE/g of the dry weight of extract was 7.33±0.04

Table 1: Total polyphenol, flavonoids and tannins content in Rosmarinus officinalis extract.
Antioxidant activity
DPPH radical scavenging assay
The antioxidant activity of the extract was evaluated in vitro by the reduction method of free radical DPPH. The results can be expressed using the parameter IC50, defined as the substrate concentration that causes a 50% loss of DPPH activity 22.
The results of the antiradical action of EQRO show an IC50 of the order of 0.128mg/ml. Our extract is less active than BHT (0.031 mg/ml).

Table 2. IC50 values of aqueous extract and BHT.
Antibacterial activity
The antibacterial activity was determined by measuring the diameter of the zone of inhibition, which was determined by the different concentrations of the EQRO around the discs. The results are recorded in Table 3 and Figure 1. This result shows that the aqueous extract only inhibits Gram (+) bacterial strains at different concentrations (A: 200, B: 400 and C: 600 mg/ml) with diameters of 10mm, 13mm, and 12mm, respectively. Rosemary is found to be inactive against Escherichia coli and Pseudomonas aeruginasa.
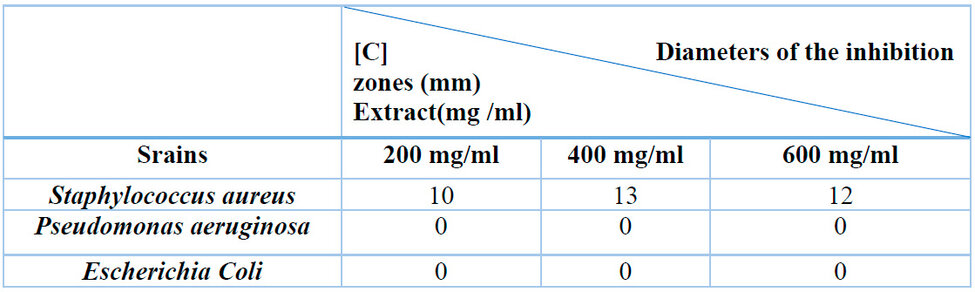
Table 3: Antibacterial test results.
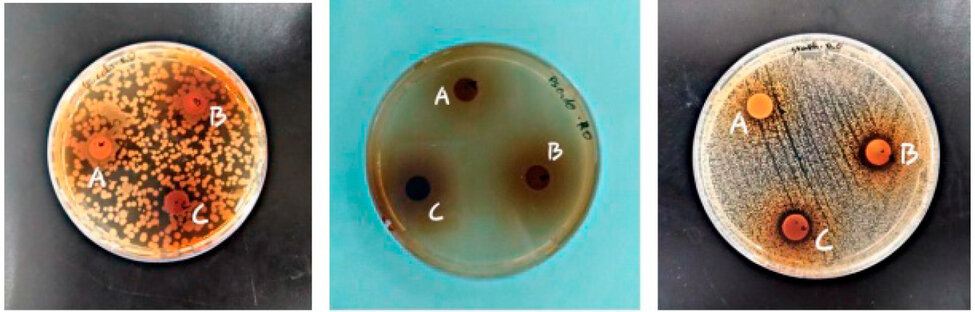
Figure 1: Growth inhibition zone of the three bacteria towards different concentrations of EQRO.
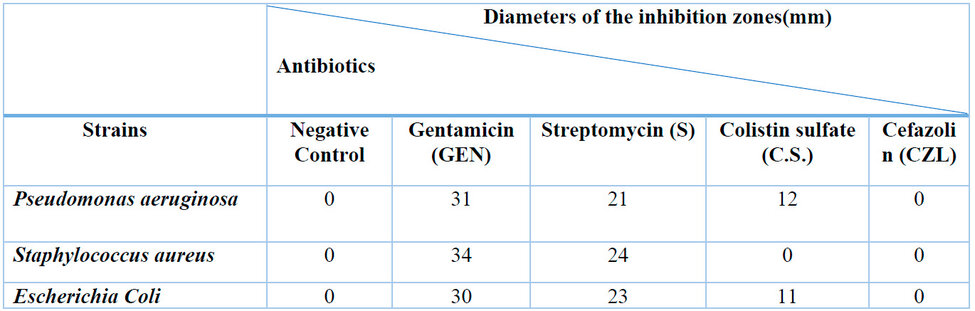
Table 4: Diameters of the antibiogram inhibition zones of the three strains tested (S. aureus, P. aeruginosa, E. coli).
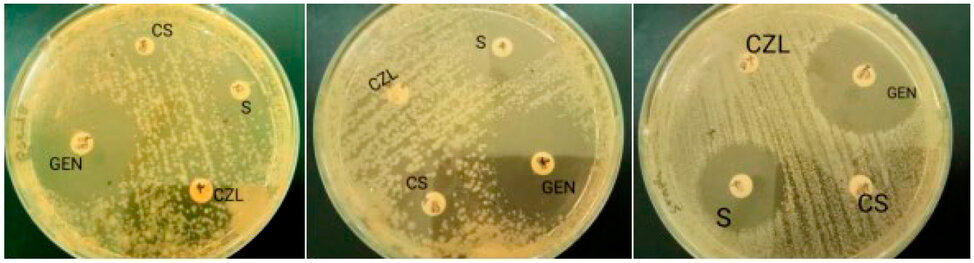
a) P. aeruginosa b) E. coli c) S. aureus
Figure 2: Antibiogram of strains: Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus.
It is observed that the bacterial strains react differently to the antibiotics tested; it is noted that the strains tested present an upper zone between 30 and 34 mm, which explains their sensitivity to gentamicin.
Cefalozine does not express any zone of inhibition on the three bacterial strains tested, and for Colistin sulfate, no zone of inhibition was found on S. aureus. This means that the bacterial strains are resistant to these antibiotics.
DISCUSSION
The aqueous extract (EQRO) is prepared by decoction (distilled water as a polar solvent). According to 23, temperature affects the yield of extraction (yield increases with heat), which shows that the higher the temperature, the greater the process of cell penetration and the solubilization of molecules by the solvent is easy 24. However, heat can lead to the degradation of thermolabile molecules 25, so the decoction was performed briefly.
In this study, the yield of this extraction is of a value of 14.49%. One similar study was performed by 26, who recorded a yield of 14.49%. Also, there has been a high yield of 24.3% of aqueous extract of Rosmarinus officinalis originating from Sudan 27, equal to 24% in a study in Finland28.
In general, the yield of the extraction method depends on several factors such as extraction time, temperature, geographical location, harvest time, climate and storage time 23. It also depends on the nature of the solvent used 29 and the extraction method applied 30. Although ethanol and methanol were better solvents than others in extracting phenolic compounds due to their polarity and good solubility for these compounds, the results proved that ethanol was the best solvent to extract the phenolic compounds, followed by methanol and, finally, water, which could explain the difference 31.
Our results revealed that the aqueous extract is rich in polyphenols and flavonoids. Studies conducted on the contents of total phenolic compounds from three regions of Turkey, which varied between 70.3 and 147.3 mg EAG/g, differ from our results. The high polyphenol content in the aqueous extract is related to the high solubility of phenols in polar solvents 32.
The content of polyphenols in EQRO is similar to that reported by 33, which is 162 mg EAG/g, and 34, which is 127 ± 3 mg AEG/g, but are superior to those obtained by 35, which is 58.1 ± 0.9 mg AEG/g and 36 which is 39.1 ± 3.6 mg AEG/g. Furthermore, 37 and 38 recorded low polyphenol content in the leaves of Rosmarinus officinalis from France with 200 mg EAG/g and 211 mg EAG/g of extract, respectively. According to the results obtained by 36, the methanolic extract of rosemary contains 60.7±1.1mg /g, which supports our results. Thirty-nine recorded that the total flavonoid content of extracts of 28 plants is related to the content of total phenolic compounds. Likewise, we found that the flavonoid content of rosemary extracts is significantly correlated with the content of polyphenols (R2 = 0.996).
Tannins are proton donors to lipid free radicals produced during peroxidation. Forty revealed an absence of tannins from the methanol extract of Rosmarinus Officinalis in all parts of the plant.
Forty-one showed the effect of pre-extraction treatment (irradiation ionizing) and the extraction solvent on the concentration of total phenolic compounds in rosemary extracts. In addition, this difference can also be explained by the extraction method of the aerial part of the plant used to determine flavonoids 42.
The antioxidant activity is determined by a decrease in the absorbance produced by the antiradical substances 43. The results are in agreement with 44,45.
Our results are lower than those reported by 46, where the IC50 values are 0.001 mg/ml and 0.05 mg/ml, respectively, for the methanolic extract and the ascorbic acid. In another study, 47 confirmed the antioxidant power of this plant in the Bechar region with inhibition percentages of around 80.70% and 79.62% for the two methanolic and aqueous extracts, respectively.
Furthermore, 48 reported that the concentration of total polyphenols is significantly correlated with the antioxidant capacity generally evaluated by the test DPPH. It is known that the antioxidant activity is solid and practical. That is, the value of IC50 is weak.
According to Turkmen 49, polyphenols are influential hydrogen donors to the DPPH radical due to their ideal structural chemistry. For the past 10 years, rosemary and its constituents (carnosol, acid carnosic acid, ursolic acid, rosmarinic acid, and caffeic acid) have been extensively studied 50. Carnosic acid and carnosol are responsible for 90% of the antioxidant activity of rosemary and together represent about 5% of the dry weight of its leaves 51,52.
The results of the antibacterial action of the rosemary extract showed a difference in the diameters of the zone of inhibition of Escherichia Coli ATCC (0.00 mm) compared to the results obtained by 53 of the ethanolic extract (16.62 mm).
Our results agree with the scientific work of 54, who mentioned that the aqueous extract of Rosmarinus officinalis is inactive on all Gram – (E. coli, P. aeruginosa) strains.
The antimicrobial activity depends not only on the presence of the phenolic compounds but also on the presence of various secondary metabolites 55, location, and number of hydroxyl groups 42.
Several classes of polyphenols, such as phenolic acids, flavonoids and tannins, serve as a defense mechanism of plants against microorganisms, insects, and pathogenic herbivores 42. Polyphenols, tannins and flavonoids such as epigallocatechin, catechin, myricetin, quercetin, 56 and Luteolin 57 are important antibacterial substances.
CONCLUSIONS
In conclusion, the EQRO of the leaves had a high content of total polyphenols, flavonoids, and tannins, which also had an antibacterial effect. This suggests that the EQRO plays a protective role from oxidative stress. Data from this study indicates that Rosmarinus officinalis can either increase antioxidant power, reduce oxidative stress, or do both. These results support the beneficial utilization of this plant as a natural antioxidant in food and folk medicine, which prevents excessive production of free radicals and is used as an antibacterial agent.
Acknowledgments: This work was supported by the Algerian Ministry of Higher Education and Scientific Research (MESRS). We express our gratitude to these organizations.
Conflicts of Interest: The authors declare no conflict of interest
Author contribution:
BENCHEIKH Dalila: Supervise, conceptualize, validate, visualize, perform formal analysis, write reviews and edits, and write original drafts.
Khawla laichi: Data curation; Formal analysis; Investigation; Methodology; Software; Writing-original draft
Chemseddine herizi: Data curation; Formal analysis; Investigation; Methodology; Software; Writing-original draft; Writing-review & editing.
Mebarka Ahmed Azi: Data curation; Formal analysis; Investigation; Methodology; Software; Writing-original draft; Writing-review & editing.
Seddik khennouf: Project administration; Validation; Visualization; Writing-review & editing
Saliha dahamna: Conceptualization; Visualization; Writing-original draft; Writing-review & editing.
REFERENCES
1. Lian, L.J.; Xu, J.;Wu, C.; Wang,X.F.; Fu, W.Y.; Xu, L.H. Lead induces oxidative stress, DNA damage and alteration of p53, Bax and Bcl-2 expressions in mice. Food Chem Toxicol. 2008, 46(5),pp.1488-94.
2. Migdal, C.;Serres, M. Espèces réactives de l’oxygène et stress oxydant. Med Sci. 2011,27(4),pp. 405-412.DOI. https://doi.org/10.1051/medsci/2011274017
3.Vanden, B.D.A; Vlietnick ,A. J.; Hostettmann, K. Screening methods for antibacterial agents from higher plants. Methods in plant Biochemistry. Assay for Bioactivity. Academic Press, London. 1991,6,pp.47–69.
4.Biyiti, L. ; Meko'o, D.; Tamzc, V. ;Amvam ,Z. Recherche de l'activité antibactérienne de quatre plantes médicinales camerounaises. Pharm Med Trad Afr. 2004,13pp. 11-20.
5.Pehlivan, M.; Mohammed, F.S.; Şabik, A.E.; Kına, E.; Dogan, M.; Yumrutaş, Ö; Sevindik, M. Some Biological activities of ethanol extract of Marrubium globosum. Turk. J.Agri-Food Sci and Tech.2021, 9(6)pp. 1129-1132. DOI: 10.24925/turjaf.v9i6.1129-1132.4382
6. Lias, F.; Kholkhal, W. Gaouar Nassira. Bekhechi Chahrazed. Bekkara Fawzia Atik. Antibacterial and antifungal Activities of olive (Olea europaea L.)from Algeria.J. Microbiol. Biotech. Res.2011. 1 (2)pp. 69-73.
7.Jamshidi-Kia, F.; Lorigooini. Z.; Amini-Khoei, H. Medicinal plants: Past history and future perspective. Journal of herbmed pharmacology. 2018,7pp.1-7
8.Pehlivan, M.; Mohammed, F.S.; Şabik, A.E.; Kına, E.; Dogan, M.; Yumrutaş, Ö.;Sevindik, M. Some Biological activities of ethanol extract of Marrubium globosum. Tur. J. Agri-Food Sci Tech. 2021, 9(6)pp. 1129-1132. DOI: 10.24925/turjaf.v9i6.1129-1132.4382
9.Begum, A.; Sandhya, S.; Syed S.A.A.; David, B. An in-depth review on the medicinal flora Rosmarinus officinalis (Lamiaceae). Acta Scientiarum Polonorum, Tech Alim. 2013,12(1)pp.61-74.
10.Mouas, Y.; Djemal, H.; Megdad,F.; Benrebiha, F.Z. Etude de l’influence de trois écotypes différents (Blida, Djelfa et Msila) sur la variation des paramètres physiologiques et biochimiques du romarin Rosmarinus officinalis L. Revue Agrobio. 2016,6 (1)pp. 96-100.
11.Reguieg, L. Using medicinal plants in Algeria. Amer J food nut .2016,1(3)pp.126-127.
12.Ibanez, E.; Kubatova, A.; Senorans, F.J ; Cavero, S.; Reglero, Guillermo, B.; Hawthorne, S. Subcritical Water Extraction of Antioxidant Compounds from Rosemary Plants.J. Agric. Food Chem. 2003,51(2)pp.375–38. DOI: 10.1021/jf025878j
13.Peng, Y. ; Yuan, J.; Liu, F.; Ye, J. Determination of active components in rosemary by capillaryelectrophoresis with electrochemical detection. J Pharm Biom Anal. 2005,pp.39: 431.
14.Chevallier, A. Encyclopédie des plantes médicinales. Larousse. 2001,p. 61, 293.ISBN. 2035602521, 9782035602527
15.Slinkard, K.; Singleton, V.L. Total phenol analysis: automation and comparison with manual methods. American Journal of Enology and Viticulture.1977,pp.28:49–55. DOI: 10.5344/ajev.1977.28.1.49
16. Sakanaka, S.; Kim, M.;; Taniguchi, M.;, Yamamoto, T.. Antibacterial substances in Japanese extract against Streptococcus mutans, a cariogenic bacterium. Agr. Biol. Chem. 1989..53,pp. 2307-2311 https://doi.org/10.1271/bbb1961.53.2307
17.Bate-Smith, E. Haemanalysis of tannins, the concept of relative astringency. Phytochemistry.1973. 12,pp. 907-912.
18.Kaviarasan, S.; Naik, G.H.; Gangabhagirathi, R.; Anuradha, C.V.; Priyadarsini, K.I. In vitro studies on antiradical and antioxidant activities of fenugreek (Trigonella Foenum graecum) seeds. Food Chem.2007.103,pp. 31-37. DOI: 10.1016/j.foodchem.2006.05.064
19.Gülüce, M.; Sokmen, M.; Daferera, D.; Agar, G.; Ozkan, H.; Kartal, N.; Polissiou, M.; Sokmen, A.; Gupta, V.; Mittal, P.; Bansal, P.; Khokra, S.L.; Kaushik, D. Pharmacological potential of Matricaria recutita-A review. Inter J pharm sci drug res. 2010.2,pp. 12-6.
20.Rahal, J.J. Novel antibiotic combinations against infections with almost completely resistant Pseudomonas aeruginosa and Acinetobacter species. Clin infec dis.2006. 43(2),pp.95-99.
21.Ponce, G.; Fritz, R. ; Del Valle, E. ; Roura, I. Antimicrobial activity of essential oils on the native microflora of organic Swiss chard. Lebensmittel-Wissenschaft und – Technologie. 2003.36,pp. 679–684.
22. Markowicz Bastos, D. H.; Saldanha, L. A.; Catharino, R. R.; Sawaya, A.C.H. F.; Cunha, I B. S.; Carvalho, P. O.; Eberlin, M. N. Phenolic Antioxidants Identified by ESI-MS from Yerba Maté (Ilex paraguariensis) and Green Tea (Camelia sinensis) Extracts. Molecules. 2007.12,pp. 423-432. DOI:10.3390/12030423
24.Albano SM, Miguel MG. 2011. Biological activities of extracts of plants grown in Portugal. Industrial Crops and Products 33:338-343.
25.Seidel V.2005. Initial and Bulk Extraction. In: Sarker , S and Gray, A . Natural products isolation. Ed, Totowa , Humana Press pp 27-37 ISBN 978-1-5-8829-447-0
26.Tsai PJ, Tsai TH, Ho SC. 2007. In vitro inhibitory effects of rosemary extracts on growth and glucosyltransferase activity of Streptococcus sobrinus, Food Chemistry 105: 311– 316. DOI:10.1016/j.foodchem.2006.11.051
27.Shama IY A, Abdullah AYA, Adam KMO, Aldai MAB, Omer AMAR, Abdelgadir WS. 2014. In vitro Antimicrobial activity of Rosmarinus officinalis leave extracts. Journal of Agri-Food and Applied Sciences2(1):15-21.
28.Dorman HJD, Hiltunen R, Tikkanen MJ. 2003. Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs .Food Chemistry 83: 255–262.
29.Zhao H, Dong J, Lu J, Chen J, Li Y, Shan L, Lin Y, Fan W Gu.2006. Effects of extraction solvent mixtures on antioxidant activity evaluation and their Références bibliographiques extraction capacity and Selectivity for free phenolic compounds in barley Hordeum vulgare L. J. Agric. Food Chemistry 54: 7277−7286.
30. Wojdylo, A., Oszmianski, J., Czemerys, R. (2007) Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 105: 940-949.
31.Mohsen Sobhy M, Ammar Abdella SM.2009. Total phenolic contents and antioxidant activity of corn tassel extracts. Food Chemistry 112(3): 595-598.
32.Yesil-Celiktas O, Girgin G, Orhan H, Wichers HJ, Bedir E, VardarSukan F. 2007. Screening of free radical scavenging capacity and antioxydant activities of Rosmarinus Officinalis extract with Focus on location and harvesting times. European food research and technology 224: 443-51.
33.Erkan N, Ayranci G, Ayranci E.2008. Antioxidant activities of rosemary (Rosmarinus Officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem 110: 76-8.
34. Ho, S.C., Tsai, T.H., Tsai, P.J., Lin, C.C. (2008) Protective capacities of certain spices against peroxynitrite-mediated biomolecular damage. Food and Chemical Toxicology. 46: 920-928.
35.Su, X. ; Duan, J. ; Jian, Y. ; Shi, J. ; Kakuda, Y. Effect of soaking conditions on the antioxidant potentials of Oolong tea’s food composition Anal.2006. 19,pp. 348- 353.
36.Tawaha, K., Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem.2007. 104(4),pp.1372-1378. DOI:10.1016/j.foodchem.2007.01.064
37.Kosar, M.; Dorman, H.J.D. ; Hiltunen, R. Effect of an acid treatment on the phytochemical and antioxidant characteristics of extracts from selected Lamiaceae species. Food Chem.2005. 91,pp.525–533.
38.Aljabri, M. Composition and antioxidant activities of rosemary (Rosmarinus officinalis) extract. Eurasian Journal of Biosciences.2020. 14,pp. 2179-2185.
39.Maisuthisakul, P.; Pasuk, S.; Ritthiruangdej, P. Relationship between antioxidant properties and chemical composition of some Thai plants. J Food Composition and Analysis.2008. 21,pp. 229-240.
40.Bouabida, N.; Benoufella –Kitous, K.; Ait Amar, S.; Medjdoub- Bensaad, F.; Graiche, F. Evaluation of biocidal activity of four Lamiaceae leaves on the black bean aphid Aphis fabae Scopoli, (Homoptera: Aphididae).Acta agriculture Slovenica.2022.118,pp.1-2212
41.Perez, M.B.; Calderon, N.L.; Croci, C.A. Radiation-induced enhancement of antioxidant activity in extracts of rosemary (Rosmarinus officinalis L.).Food Chem.2007. 104,pp. 585-592.
42.Fellah, H.; Ksouri, R.; Chaieb, K.; Karray, N.; Trabelsi, N.; Boulaaba, M.; Abdelly, C. Phenolic composition of Cyinara Cardunculus L. Organs, and their biological activities. Compte rendu biologie. 2008.331,pp. 372-379.
43.Talbi, H.; Boumaza, A.; El-mostafa, K.; Hilali, A. Evaluation de l’activité antioxydant et la composition physico-chimique des extraits méthanoliques et aqueux de la Nigella sativa L. Sci.2015.6(4) ,pp.1111-1117.
44.Tepe, B.; Sokmen, M.; Akpulat, H.A.; Sokmen, A. Screening of the antioxidant potentials of six Salvia species from Turkey. Food Chem.2006. 95,pp. 200-204.
45.Kivilompolo, M.; Hyotylainen, T. Comprehensive two-dimensional liquid chromatography in analysis of Lamiaceae herbs: Characterisation and quantification of antioxidant phenolic acids. J Chromatography A. 2007.1145,pp. 155-164. DOI: 10.1016/j.chroma.2007.01.090
46. Fadili, K.; Amalich, S.; N’dedianhaoua, S.K.; Bouachrine ,M.; Mahdjoubi, M.;EL HILALI, F.; Zair, T.2015. Polyphenols content and antioxidant activity of two species from Moroccan High. Inter J Innov Sci Res.2015. 17 ( 1),pp .24-33
47.Makhloufi, A. Antibacterial Activity of the Extracts Obtained from Rosmarinus officinalis, growin in wild in Bechar region, south west of Algeria. Appl Biol Sah Areas.2017. 1( 2),pp.30-36.
48.Damak, N.; Bouaziz, M.; Ayadi, M.; Sayadi, S.; Damak, M. Effect of the Maturation Processon the Phenolic Fractions.Fatty Acides,and Antiioxidant Activity of the Chétoui Olive Fruit Cultivar.Agric.Food Chem.2008. 56,pp.1560-1566. DOI: 10.1021/jf072273k
49.Turkmen, N.; Velioglu, Y.S.; Sari, F. ; Polat, G. Effect of Extraction Conditions on Measured Total Polyphenol Contents and Antioxidant and Antibacterial Activities of Black Tea. Molec.2007. 12,pp.484-496. DOI: 10.3390/12030484
50.Slamenova, D.; Kuboskova, K.; Horvathova, E.;Robichova, S. Rosemary-stimulated reduction of DNA strand breaks and FPGsensitive sites in mammalian cells treated with H2O2 or visible light-excited Methylene Blue.Cancer Letters.2002. 177,pp. 145-153. DOI: 10.1016/S0304-3835(01)00784-4
51.Wei, G.J.; Ho, C.T. A stable quinone identified in the reaction of carnosol, a major antioxidant in rosemary, with 2, 2-diphenyl-1-picrylhydrazyl radical. Food Chem.2006. 96,pp. 471- 476. DOI: 10.1016/j.foodchem.2005.02.041
52.Visanji, J.M.; Thompson, D.G.; Padfield, P.J. 2006. Induction of G2/M phase cell cycle arrest by carnosol and carnosic acid is associated with alteration of cyclin A and cyclin B1 levels. Cancer Letters.2006.237,pp. 130-136. DOI: 10.1016/j.canlet.2005.05.045
53.Zhang, L.; Abbott, J.J.; Dong, L.; Kratochvil, B.E.; Bell, D.; Nelson, B. J. Artificial bacterial flagella: Fabrication and magnetic control. Appl Phys Letters.2009. 94(6),pp. 064-107.
54.Balouiri,M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity. Journal of Pharmaceutical Analysis.2016. 6(2).pp , 71-79.
55.Kil, H.Y.; Seong, E.S.; Ghimire, B.K.; Chung, I.M.; Kwon, S.S.; Goh, E.J.; Heo, K.;Kim, M. J.; Lim, J.D.; Lee, D.; Yu,C.Y. Antioxidant and antimicrobial activities of crude sorghum extract.Food Chem.2009. 115(4),pp. 1234-1239.
56.Shan, B.; Cai, Y.Z.; Brooks, J.D.; Corke, H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Inter J Food Micr.2007.117,pp.112-119.DOI:10.1016/j.ijfoodmicro.2007.03.003
57.Askun, T.; Tumen, G.; Satil, F. Ates M. In vitro activity of methanol extracts of plants used as spices against Mycobacterium tuberculosis and other bacteria. Food Chem. 2009.116,pp. 289-294. DOI: 10.1016/j.foodchem.2009.02.048
Received: October 9th 2023/ Accepted: January 15th 2024 / Published:15 February 2024
Citation: Bencheikh D., Laichi K., Herizi C., Azi M. A., Khennouf S., Dahamna S. Rosmarinus Officinalis: Phytochemical analysis and biological activities. Bionatura Journal 2024; 1 (1) 35. http://dx.doi.org/10.21931/BJ/2024.01.01.35
Additional information Correspondence should be addressed to [email protected]
Peer review information. Bionatura Journal thanks anonymous reviewer(s) for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
All articles published by Bionatura Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Publisher's Note: Bionatura Journal stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2024 by the authors. They were submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
