2024..01.01.59
Files > Conference Series > 2024 > Chimboazo ild pagina nueva
Influence of a natural colorant powder from Syzygium cumini L. (Skeels) on sensory and physicochemical properties during storage of a heat-treated flavored fermented milk
Fabián M. Gaibor1, Daliannis Rodríguez2, Mario A. García3*, Alicia Casariego4
1Yachay University for Experimental Technology and Research, Imbabura, Ecuador; [email protected].
2Dept. of Food, Pharmacy and Food Institute, University of Havana, Cuba; [email protected].
3La Maná Extension, Faculty of Agricultural Sciences and Natural Resources, Technical University of Cotopaxi, Ecuador; [email protected].
4Dept. of Food, Pharmacy and Food Institute, University of Havana, Cuba; [email protected].
* Correspondence: [email protected]; Tel.: +593 995537689
Available from. http://dx.doi.org/10.21931/BJ/2024.01.01.59
ABSTRACT
This study aimed to evaluate the influence of a powder colorant obtained from a hydroalcoholic extract of jambolan (Syzygium cumini) on the chemical, microbiological, and sensory characteristics of heat-treated flavored fermented milk. The extraction of anthocyanins from the pulp was carried out by maceration with 90% (v/v) ethanol acidified with citric acid. This extract was concentrated (14 to 15% w/v of total solids). Maltodextrin DE 12 was added to obtain 25% (w/v) total solids. Guar gum (0.06% w/w) was added as a stabilizer to whole milk reconstituted with sterilized distilled water (11% w/v total solids). The colorant powder was homogenized at a rate of 1.5 and 2 g per 100 mL of powdered whole milk dissolved in the corresponding amount of water, and sucrose and concentrated strawberry flavoring were added. The natural colorant addition allowed us to obtain a product with pH (4.23-4.75), titratable acidity (1.28-1.47% w/w lactic acid), and color stability, similar to those of yogurt with synthetic colorants. No microbial growth or color changes were detected. The judges did not notice any strange odor, taste, or color. Natural colorants can be a beneficial option for developing healthy and sustainable foods.
Keywords: Syzygium cumini; anthocyanins; spray-dried; natural colorant; heat-treated fermented milk.
INTRODUCTION
Consumer trends focus on consuming fresh foods or processed foods that offer some health benefits.1 To meet these demands, many native or little-explored fruits have been evaluated as raw materials for the food industry.2
Syzygium cumini (L.) Skeels is one of these fruits, known in various places as jambolan. When ripe, it has an intense purple color that is very attractive for industrial processing. Its ripe fruits, with a high anthocyanin content,2 is used to produce juices, nectars, and jams.3 Interest in anthocyanin pigments in scientific research has increased in recent years due to the color they impart to products that contain them and their possible health benefits.4 Therefore, besides their functional role, anthocyanins as natural pigments constitute potential agents for obtaining value-added products for human consumption.
Anthocyanins are phenolic compounds from the flavonoid family, widely distributed in nature.5 They are water-soluble, facilitating their incorporation into many aqueous food systems. This quality makes these pigments attractive natural colorants6 for preparing foods such as heat-treated flavored fermented milk.
Milk and dairy products are important foods for people of any age. Along with milk, fermented milk has reached a higher proportion in the daily diet of modern society due to its nutritional value and sensory characteristics. In the United States of America, between 2000 and 2023, per capita yogurt consumption experienced growth of more than 50%.7 Of all dairy products, fermented milk is well known and the most popular around the world6.
Some researchers have examined the elements that impact the stability of anthocyanins in fruit products. However, information on the factors influencing the stability of anthocyanins during storage of fruit yogurts is limited.8 Furthermore, in most cases, fruit pulps or extracts rich in anthocyanins are added, but not natural powdered colorants. This work evaluated the influence of adding a colorant powder obtained by spray drying from a hydroalcoholic extract of jambolan (S. cumini) on some physical, chemical, and sensory characteristics of heat-treated flavored fermented milk. The novelty of this work lies in the incorporation of a powdered colorant explicitly obtained from the jambolan, which expands the sources of anthocyanins studied as alternatives to synthetic colorants in flavored fermented milks, demonstrating their effectiveness for color without altering the physical, chemical and sensory properties, opening new possibilities of industrial application that take advantage of the nutraceutical potential of this natural pigment.
MATERIALS AND METHODS
Raw material
Ripe jambolan fruits were harvested between September and November 2022 in Havana, Cuba, considering similarities in size, color, uniform ripeness, and absence of visible defects. The pulp and skin were ground in an IKA T25 digital Ultra-Turrax (model T25 D S25).
Chemical reagents and other materials
For the extraction of anthocyanins and their evaluation, absolute ethanol (Sigma-Aldrich, St. Louis, MO, USA), citric acid food grade (Solvesa, Ecuador), Folin-Ciocalteu reagent (Sigma-Aldrich, St. Louis, MO, USA), and potassium chloride and sodium acetate (Merck KGaA, Darmstadt, Germany) as buffers were used. Maltodextrin DE 12 (Glucidex®, Roquette America, Inc., USA) was used as a spray drying aid. In the production of fermented milks whole milk powder (La Vaquita, Ecuador), guar gum (Merck KGaA, Darmstadt, Germany) as a stabilizer, sucrose (Sigma-Aldrich, St. Louis, MO, USA), strawberry flavor concentrate (Esencias Levapan® Fresa, Ecuador), yogurt starter culture (YC280, Ferlact Biotech, Ecuador), commercial colorant (Colorantes Levapan® Rojo, Ecuador) were used. Sabouraud dextrose agar (Sigma-Aldrich, St. Louis, MO, USA) was used as the culture medium, and in the determinations of titratable acidity, sodium hydroxide solution (1.0 N, Sigma-Aldrich, St. Louis, MO, USA) and phenolphthalein (Sigma-Aldrich, St. Louis, MO, USA). In addition, distilled water was needed.
Preparation of anthocyanin extract
The extraction of anthocyanins from the pulp was carried out by maceration with a pulp/ethanol dissolution ratio of 1 g per 5 mL of 90% (v/v) ethanol acidified with 0.03% (w/v) citric acid. The mixture was kept at room temperature for 6 h on a sieve (Retomed, Mizard. 2001) at 285 rpm. After that time, the solid residue was filtered and discarded.9 The polyphenolic compounds, expressed as gallic acid in mg/100 g, were quantified with the Folin-Ciocalteu reagent,10 and the anthocyanin content was determined by the pH differential method11 and was expressed as cyanidin-3- glucose with a molar extinction coefficient of 26900 L/cm mol and molar mass of 449.2 g/mol.9 In this determination, buffer potassium chloride solutions were used at 0.025 M (pH 1.0, adjusted with HCl) and sodium acetate at 0.4 M (pH 4.5). This diluted hydroalcoholic extract determined the extraction yields of total polyphenols and anthocyanins and pH (Mod. Basic 20, Crison, España)12. Both yields (%) were calculated as the ratio between the contents of the extract and the pulp.9
Spray drying
The diluted hydroalcoholic extract was concentrated to a total solids content between 14 and 15 % in a rotary evaporator (IKA, Germany) at 40 °C with a rotation of 85 rpm, vacuum pump (ULVAC DTC-21). In addition, the concentrated extract was determined by its density using a capillary tube pycnometer,13 soluble solids in an Abbe refractometer with temperature correction, and total solids with a thermogravimetric balance (Sartorius, Göttingen, Germany) until constant mass at 105 ºC.12
Maltodextrin DE 12 was added to 50 mL of the concentrated extract for a final total solids content of 25% (w/v). Drying was carried out in a spray dryer, Mod. B-191 (BÜCHI, Switzerland) has a 0.7 mm diameter nozzle and 60 m3h-1 of airflow when the aspirator is 100 %. The inlet air temperature was guaranteed by preheating the equipment for 15 min. The outlet temperature was kept constant at 70 ºC. The feed rate was 65 % (963 mL/h) for 165 ºC.14
Preparation of the heat-treated flavored fermented milk
The milk was prepared by reconstitution of whole milk powder with sterilized distilled water to obtain a product with 11% (w/v) of total solids, and guar gum (0.06% w/w) was added. The mixture was pasteurized at 90 ºC for 15 min. Once this process was finished, samples were cooled until 42 ºC and the powdered colorant was added and homogenized under hygienic conditions at a rate of 1.5 and 2 g per 100 mL of whole milk powder dissolved in the amount of water followed by sucrose and 0.016% (v/v) strawberry flavor concentrate (Esencias Levapan® Fresa, Ecuador). They were then inoculated with 2% (w/v) yogurt starter culture (YC280, 2x1012 CFU/mL). During fermentation (42 ºC), the pH was measured at 30-minute intervals until reaching values around 4.5 to 4.7. Once the fermentation was finished, the product was pasteurized. The samples were then cooled to 5 ºC and packed in 150 mL amber glass bottles.
The maximum concentration of powdered natural colorant to be added to flavored fermented milk (1.5 and 2 g of powder per 100 mL) was determined through an acceptance/rejection test in focus group sessions where 10 semi-trained judges participated.15 The judges visually indicated that the coloration was similar to commercial strawberry fermented milk.
To compare the changes in microbiological, chemical, and sensory indicators during storage, a control heat-treated fermented milk was prepared with the addition of a commercial colorant (Colorantes Levapan® Rojo, Ecuador). All samples were stored between 4 and 5 ºC for 23 days.
Microbiological and chemical determinations
As part of the microbiological control, counting molds and yeasts16 was carried out on Sabouraud agar. These cultures were incubated at a controlled temperature of 25 °C for a standard incubation period of 5 days, allowing the formation and development of microbial colonies. Counting was performed using serial dilution techniques to ensure the accuracy of the results.
In addition, the pH (Mod. Basic 20, Crison, España) and titrable acidity17 in the yogurt were evaluated. The pH was measured using a calibrated electrode immersed directly in the yogurt samples at 25°C. The determination of acidity, expressed as a percentage of lactic acid, was measured using neutralization titration. All measurements were performed in triplicate to ensure reproducibility of the results.
Sensory evaluation
The quantitative descriptive analysis used a balanced block design to evaluate the sensory evaluation. The sensory descriptors (characteristic color, strange color, smell of fermented product, fruity smell, peculiar smell, distinctive flavor, strange flavor, sweetness, acidity, fruity flavor, viscosity, and overall quality), obtained by the controlled association method,18 were evaluated on a structured scale, bounded at both ends with increasing intensity of the descriptor, from left to right. Ten semi-trained judges evaluated the samples.
Statistical analysis
Variance analysis was performed, and in the event of significant differences, Duncan's multiple range test was applied to determine the differences with a confidence level of 5%. The STATISTICA program (version 7, 2004, StatSoft. Inc., Tulsa, USA) was used.
RESULTS AND DISCUSSION
Characterization of diluted hydroalcoholic extract and concentrated extract
The extraction process presented yields of total polyphenols and anthocyanins of 73 and 31%, respectively, with a density of 0.8824 g/mL, pH of 4.2, and 2.7% (w/v) of total solids. The jambolan extract concentrated by rotary evaporation was evaluated before the addition of maltodextrin DE 12. The anthocyanin content was 66.91 mg/100 g, and the total polyphenol content was 1781.32 mg/100 g. The percentage values of total solids, density, and soluble solids corresponded to 14.64%, 1.1033 g/mL, and 20.73 ºBrix, respectively. Similar results of 60.57 mg/100 g of anthocyanins, 82.59 % humidity, 17.41 % total solids, and 20 ºBrix were reported for jabuticaba extracts.19
The behavior of the pH values of heat-treated flavored fermented milk during storage
Table 1 presents the behavior of the pH values of flavored fermented milk during storage between 4 and 5 ºC. At the beginning of storage, the fermented milk with the natural powdered colorant showed significant differences from the control fermented milk. However, the pH values ranged between 4.23 and 4.75, corresponding with this product type.20 The products with the addition of the natural powdered colorant had a lower pH value (p ≤ 0.05) than that of the control fermented milk, which could be due to the acidity provided by the powder (~4.5).
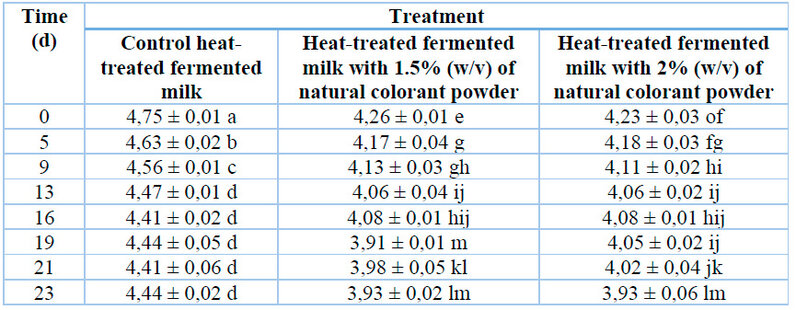
Mean ± standard deviation; n= 3.
Different letters indicate significant differences (p ≤ 0.05).Table 1: The behavior of the pH of heat-treated flavored fermented milk during refrigerated storage.
At the end of storage, all the formulations presented pH values in the recommended range (3.6 to 4.5) in the Brazilian technical regulation of identity and quality of fermented milk of normative instruction No. 46 that establishes pH values for identity and quality.21
In a study 22 about the effect of Hibiscus sabdariffa (Calyce) extract on the chemical and organoleptic properties of yogurt, a pH of 4.3 was obtained. In contrast, in another work,23, a pH of 4.6 was reported for yogurt with a natural colorant rich in anthocyanins extracted from strawberries (Fragaria × ananassa).
The behavior of the acidity values of heat-treated flavored fermented milk during storage
The behavior of the acidity corresponded, in a general way, with the variation of the pH during storage (Figure 1). In the beginning, the acidity of the control-fermented milk was 0.97%, while for the fermented milk with 1.5 and 2% (w/v) of powdered natural colorant, the values were 1.28 and 1.47, respectively. These last values were similar to those reported for yogurt with microencapsulated anthocyanins extracted from Solanum melongena L. Bark.24
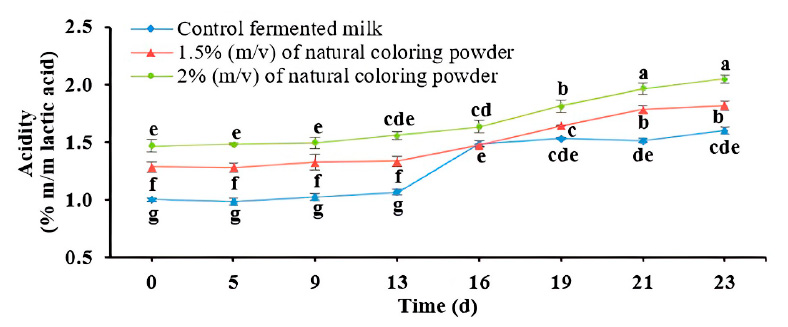
Figure 1: Acidity behavior of heat-treated flavored fermented milk during refrigerated storage. Error bars indicate the standard deviation. Different letters indicate significant differences (p ≤ 0.05).
Initially, in the control product and the fermented milk flavored with natural colorant, the acidity was by the quality specifications, which established a minimum ideal acidity expressed as lactic acid, 0.3% for yogurt.25 The titratable acidity for all the flavored fermented milk increased as the powdered colorant content increased during storage. This behavior of acidity during storage corresponded to what was reported.24
Microbiological indicators
The products of the three treatments met the sanitary requirements in correspondence with the limits specified in the NTE-INEN 239520 for fermented milk. During storage, mold and yeast growth was detected in 19 days in all cases, with counts between 2 and 3 CFU/mL maintained until 23 days of storage. According to these results, the microbiological quality for the three formulations during the 23 days of storage was satisfactory, complying with what is established for this type of product.
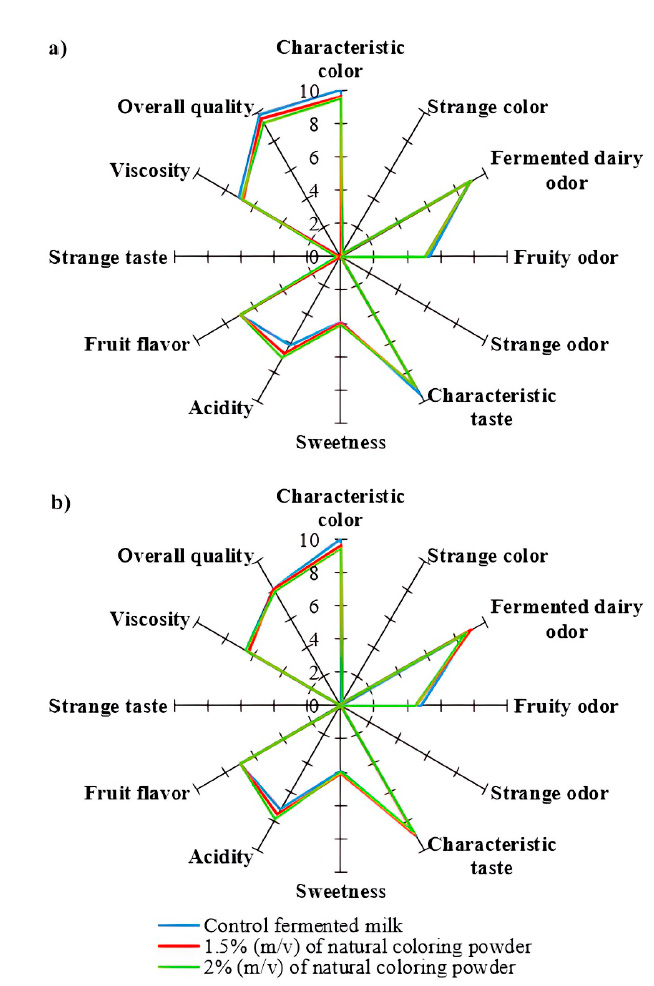
Figure 2: Sensory descriptors evaluated in the heat-treated flavored fermented milk (a) at the beginning of the storage; (b) at 23 days of storage between 4 and 5 ºC.
Sensory evaluation
Figure 2 shows some of the sensory descriptors evaluated during storage. For all the treatments, the tasters thought that products with an odor, acidity, and viscosity characteristic of this fermented product were obtained. The color appeared uniformly and homogeneously. As can be seen in the figure, the order of addition of the natural colorant did not influence the perception of the color of the product. However, there is evidence suggesting that the timing of placing the natural colorant from fruits can affect the stability of anthocyanins during fermentation. Studies, such as the one on fruit yogurt, indicate that adding anthocyanin extract before the fermentation of milk could impact the pigment content in yogurt, affecting its color stability during storage.8 Additionally, research on fermented plant-based products, like potato blueberry yogurt, points out that adding fruit preparations before fermentation can cause color changes, impacting sensory properties and physicochemical parameters.26
No lumps were observed, indicating that the lactic acid bacteria acidified the medium quickly and correctly, so there were no defects in the microstructure; therefore, adding the colorant to the flavored fermented milk did not affect its development. Lactic acid bacteria significantly contribute to the effective acidification of the matrix and rapidly consume sugars during fermentation.27
They also reported positive criteria regarding flavor-related descriptors, typical of a fermented milk product and characteristic of fruit. The sweetness was adequate. The tasters did not detect the presence of strange color, odor, or flavor in the recently prepared products.
At the end of storage, the tasters scored with a higher value for the acidity of all the treatments, corresponding with the variation of the titratable acidity (Figure 1) and pH (Table 1). In general, the scores given to the sensory descriptors decreased due to the loss of characteristics of a fresh fermented product. Although the overall quality rating of the three treatments could be considered `very good,' the increase in acidity caused an increase in the flavor of coagulated sour milk and the smell of the fermented product, related to the occurrence of the fermentation process during storage.
A result of interest for the study was related to the fact that the tasters did not detect changes in the color of the products during their storage, even in treatments with the addition of natural powdered colorant. The increase in acidity during storage could favor the development and stability of the characteristic color expected for these strawberry-flavored fermented kinds of milk. Anthocyanins have more excellent stability in aqueous solutions at acid pH.28 It has been suggested that a high pH accelerates the degradation of pigments during storage. In contrast, slight variations in the pH of the medium did not affect the stability of the anthocyanins.29
CONCLUSIONS
The addition of natural colorant allowed fermented milk with a flavor and a pH (4.23-4.75), titratable acidity (1.28-1.47% w/w lactic acid), and color stability similar to those of fermented milk with synthetic colorants. No microbial growth or color changes were detected. The judges did not notice any strange odors, flavors, or colors. The use of natural colorants in the food industry is a beneficial option for both human health and the environment, and its use can contribute to the development of healthier, more attractive, and sustainable food products.
Author Contributions: Conceptualization, F.G. and D.R.; methodology, F.G.; software, M.G.; validation, M.G. and A.C.; formal analysis, F.G. and D.R.; investigation, F.G. and D.R.; data curation, M.G.; writing—original draft preparation, F.G. and D.R.; writing—review and editing, M.G.; visualization, A.C.; supervision, A.C.
Funding: This research received no external funding.
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Not applicable.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Rodrigues, N.R.; Pinheiro, R.; Flávio, F. Jambolan (Syzygium cumini (L.) Skeels): A review on its nutrients, bioactive compounds and health benefits. J. Food Compos. Anal. 2022, 109, 104491. https://doi.org/10.1016/j.jfca.2022.104491
2. Qamar, M.; Akhtar, S.; Ismail, T.; Wahid, M.; Abbas, M.W.; Mubarak, M.S.; Yuan, Y.; Barnard, R.T.; Ziora, Z.M.; Esatbeyoglu, T. Phytochemical profile, biological properties, and food applications of the medicinal plant Syzygium cumini. Foods 2022, 11(3), 378. https://doi.org/10.3390/foods11030378
3. Ayyanar, M.; Subash-Babu, P. Syzygium cumini (L.) Skeels: a review of its phytochemical constituents and traditional uses. Asian Pac. J. Trop. Biomed. 2012, 2(3), 240-6. https://doi.org/10.1016/S2221-1691(12)60050-1
4. Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61(1), 1361779. https://doi.org/10.1080/16546628.2017.1361779
5. Tonon, V.; Brabet, C.; Pallet, D.; Brat, P.; Hubinger, D. Physicochemical and morphological characterization of açai powder produced with different carrier agents. Int. J. Food Sci. Technol. 2009, 44(10), 1950-58. https://doi.org/10.1111/j.1365-2621.2009.02012.x
6. Gangwar, R.; Hai, H.A.; Kumar, P.; Sharma, N.K. Development and quality evaluation of yoghurt fortified with pineapple, apple and sweet lemon juice (Fruit Yoghurt). Int. J. Eng. Res. Technol. 2016, 5(3), 621-9. https://doi.org/10.17577/IJERTV5IS030484
7. USDA-ERS. Dairy Data. Available online: https://www.ers.usda.gov/data-products/dairy-data.aspx (accessed on 15 January 2024).
8. Ścibisz, I.; Ziarno, M. Effect of fermented matrix on the color and stability of strawberry and blueberry anthocyanins during the storage of fruit yogurts and soy-based and bean-based fruit yogurt alternatives. Molecules 2023; 28(17), 6222. https://doi.org/10.3390/molecules28176222
9. Gaibor, F.M.; Cuba, A.; Rodríguez, D.; García, M.A.; Casariego, A. Optimización del proceso de extracción hidroalcohólica a partir de pulpa de cerezo negro (Syzygium cumini L. Skeels). Cienc. Tecnol. Aliment. 2017, 27(2), 51-9.
10. Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteau reagent. Method. Enzymol. 1999, 299, 152-78. https://doi.org/10.1016/S0076-6879(99)99017-1
11. Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J. AOAC Int. 2005, 88(5), 1269-78. PMID: 16385975.
12. Gaibor, F.M.; Rodríguez, D.; Fundora, L.; Salas, E.; Rodríguez, J.L.; Falco, A.S.; Casariego, A.; García, M.A. Evaluación de las características físicas, químicas, toxicológicas, antibacterianas y sensoriales del cerezo negro (Syzygium cumini L.). Cienc. Tecnol. Aliment. 2016, 26(2), 62-8.
13. Mercali, G.D.; Ribeiro, J.; Pez, D.; Tessaro, I.C.; Ferreira, L.D. Physical properties of acerola and blueberry pulps. J. Food Eng. 2011, 106(4), 283-289. https://doi.org/10.1016/j.jfoodeng.2011.05.010
14. Gaibor, F.M.; Rodríguez, D.; García, M.A.; Peraza, C.M.; Vidal, D.; Nogueira, A.; Casariego, A. Development of a food colorant from Syzygium cumini L. (Skeels) by spray drying. J. Food Sci. Technol. 2022, 59, 4045-55. https://doi.org/10.1007/s13197-022-05454-9
15. Powell, R.A.; Single, H.M.; Lloyd, K.R. Focus groups in mental health research: enhancing the validity of user and provider questionnaires. Int. J. Soc. Psychiatry 1996, 42(3), 193-206. https://doi.org/10.1177/002076409604200303
16. NTE-INEN 1529-10. Control microbiológico de los alimentos. Mohos y levaduras viables. Recuentos en placa por siembra en profundidad. Ecuador; 1998.
17. AOAC. Official Methods of Analysis. 21rst Edition. Association of Official Analytical Chemists, Gaithersburg, Maryland, USA; 2019.
18. Damasio, M.H.; Costell, E. Análisis Sensorial Descriptivo. Generación de descriptores y selección de catadores. Rev. Agroquim. Tecnol. Alim. 1991, 2, 165-78.
19. Silva, P.; Stringheta, P.; Teófilo, R.; Nolasco, I. Parameter optimization for spray-drying microencapsulation of jaboticaba (Myrciaria jaboticaba) peel extracts using simultaneous analysis of responses. J. Food Eng. 2013, 117(4), 538-44. http://dx.doi.org/10.1016/j.jfoodeng.2012.08.039
20. NTE-INEN 2395. Leches fermentadas. Requisitos. Ecuador; 2011.
21. Ministério da Pecuária, Agricultura e Abastecimento. Instrução Normativa n° 46, de 23 de outubro de 2007, Regulamento Técnico de Identidade e Qualidade de Leites Fermentados. Brasil. Available online: http://www.cidasc.sc.gov.br/inspecao/files/2012/08/instru%C3%87%C3%83o-normativa-n%C2%BA-46-de-23-de-outubro-de-2007.pdf (accessed on 15 January 2024).
22. Iwalokun, B.A.; Shittu, M.O. Effect of Hibiscus sabdariffa (Calyce) extract on biochemical and organoleptic properties of yogurt. Pak. J. Nutr. 2007, 6(2), 172-82. https://doi.org/10.3923/pjn.2007.172.182
23. Benchikh, Y.; Aissaoui, A.; Allouch, R.; Mohellebi, N. Optimising anthocyanin extraction from strawberry fruits using response surface methodology and application in yoghurt as natural colorants and antioxidants. J. Food Sci. Technol. 2021, 58(5), 1987-95. https://doi.org/10.1007%2Fs13197-020-04710-0
24. Barretto, F.J.F.P.; Clemente, H.A.; Santana, A.L.B.D.; Vasconcelo, M.A.S. Stability of encapsulated and non-encapsulated anthocyanin in yogurt produced with natural dye obtained from Solanum melongena L. Bark. Rev. Bras. Frutic. 2020, 42(3), e-137. http://dx.doi.org/10.1590/0100-29452020137
25. CXS 243. Norma para leches fermentadas. Codex Alimentarius. Food and Agriculture-United Nations Organization. World Health Organization; 2003.
26. Li, M.; He, Z.; He, L.; Li, C.; Tao, H.; Ye, C.; Liu, L.; Zeng, X.; Ran, G. Effect of fermentation parameters on the anthocyanin content, sensory properties, and physicochemical parameters of potato blueberry yogurt. Fermentation 2022, 8, 489. https://doi.org/10.3390/fermentation8100489
27. Faria-Oliveira, F.; Diniz, R.H.S.; Godoy-Santos, F.; Piló, F.B.; Mezadri, H.; Castro, I.M.; Brandão, R.L. The role of yeast and lactic acid bacteria in the production of fermented beverages in South America. In Food Production and Industry; Amer, A.H., Ed.; IntechOpen: London, 2015; pp. 107-35. http://dx.doi.org/10.5772/60877
28. Dangles, O.; Fenger, J.A. The chemical reactivity of anthocyanins and its consequences in Food Science and Nutrition. Molecules 2018, 7, 23(8), 1970. https://doi.org/10.3390/molecules23081970
29. Duan, C.; Xiao, X.; Yu, Y.; Xu, M.; Zhang, Y.; Liu, X.; Dai, H.; Pi, F.; Wang, J. In situ Raman characterization of the stability of blueberry anthocyanins in aqueous solutions under perturbations in temperature, UV, pH. Food Chem. 2024, 431, 137155. https://doi.org/10.1016/j.foodchem.2023.137155
Received: October 9th 2023/ Accepted: January 15th 2024 / Published:15 February 2024
Citation: Gaibor, F.M.; Rodríguez, D.; García, M.A.; Casariego, A. Influence of a natural colorant powder from Syzygium cumini L. (Skeels) on sensory and physical-co-chemical properties during storage of a heat-treated flavored fermented milk. Revis Bionatura 2024; 1 (1) 59. http://dx.doi.org/10.21931/BJ/2024.01.01.59
Additional information Correspondence should be addressed to [email protected];
Peer review information. Bionatura thanks anonymous reviewer(s) for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
All articles published by Bionatura Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Publisher's Note: Bionatura stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2024 by the authors. They were submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
