2024..01.01.73
Files > Conference Series > 2024 > Chimborazo
Evaluating the protective effect of alpha-lipoic acid against gentamicin-induced gonadal toxicity indicated by histopathology.
Hozaifa Elsawah1*, Aziza Amin2, Haitham Mokhimar3, Mohamed Kandiel4, Ayman Farid5, and AbuBakr El-Mahmoudy6
1Department of Pharmacology, Faculty of Veterinary Medicine, Benha University, Egypt.
2Department of Pathology, Faculty of Veterinary Medicine, Benha University, Egypt.
3Department of Pharmacology, Faculty of Veterinary Medicine, Benha University, Egypt.
4Department of Theriogenology, Faculty of Veterinary Medicine, Benha University, Egypt. [email protected].
5Department of Clinical Pathology, Faculty of Veterinary Medicine, Benha University, Egypt.
6Department of Pharmacology, Faculty of Veterinary Medicine, Benha University, Egypt. [email protected].
* Correspondence: [email protected]. Postcode: 13736, Tel.: 0020132461411, Fax: 0020132463074.
Available from. http://dx.doi.org/10.21931/BJ/2024.01.01.73
ABSTRACT
Gentamicin induces gonadotoxicity in animal models, accompanied by oxidative stress. This study evaluated the histopathological protective effect of alpha-lipoic acid against gentamicin-induced gonadotoxicity. A parallel experimental study included 50 albino Wister rats, which were divided into 5 groups of ten according to the intraperitoneal intervention: group I received gentamicin, group II received gentamicin plus alpha-lipoic (100 mg/kg), group III received gentamicin plus a double dose of alpha-lipoic acid, group III received gentamicin plus oral vitamin E, and group IV received NaCl 0.9% (control). The animals were equally euthanized on days 15 and 16. Tests were dissected, prepared, and stained using a light microscope for histopathological examination. Rats exposed to gentamicin showed degeneration of seminiferous tubules, characterized by a significant decrease in germinal epithelial cells, impaired spermatogenesis, and a lack of spermatozoa in the lumen. An improvement in testes of animals co-treated with alpha-lipoic acid or vitamin E, including restoration of spermatogenesis and epithelial thickness. The histometric analysis also showed that the tubule epithelial thickness decreased when gentamicin was used, but this did not happen when alpha-lipoic acid or vitamin E was added simultaneously. The detrimental effects of gentamicin on testicular architecture are preventable through alpha-lipoic acid or vitamin E co-treatment.
Keywords: Alpha-lipoic acid; Gonads; Histometric; Gentamicin; Seminiferous tubule; Spermatogenesis.
INTRODUCTION
Infertility is a significant public health problem affecting 8–12% of couples worldwide; approximately 40–50% is due to male factor 1. Some drugs can induce testicular dysfunction and interfere with spermatogenesis, such as gentamicin 2. Gentamicin is an aminoglycoside antibiotic widely used to treat many infectious diseases, including early-onset sepsis in neonates 3, urinary tract infections 4, and pneumonia 5. Gentamicin decreased testicular weight, reduced sperm count, motility, and viability, and increased sperm head and tail abnormalities 6, 7. These effects were associated with increased oxidative stress and altered histopathological structure 8, 9. Oxidative stress was suggested as a possible mechanism of gentamicin-induced testicular damage 10. There are many different ways that gentamicin can cause oxidative stress. Some of these are mitochondrial pathway-dependent oxidative stress 11, peroxidation of phosphoinositide by the gentamicin-iron complex 12, and lower levels of antioxidant enzymes in cells 10. Noteworthy, oxidative stress is a significant cause of male infertility through damaging spermatogenic cells 13, as the sperm plasma membrane and testis tissue are rich in polyunsaturated fatty acids, which generally provide the sperm cells with the normal motility required for capacitation 14. These polyunsaturated fatty acids are highly vulnerable to oxidative damage 15.
The alteration in testicular histopathological structure evaluated by a light microscope revealed intertubular space expansion with vacuolization, seminiferous tubule degeneration, and spermatogenic cell depletion 2, 9. Moreover, cellular changes were also reported with a transmission electron microscope in rats treated with gentamicin, including primary spermatocytes and Sertoli cells 9. Alpha-lipoic acid is a powerful antioxidant coenzyme that showed histopathological protection against adriamycin-induced testicular damage 16. Also, it restored gentamicin-induced testis weight reduction, normalized gentamicin-induced impaired sperm parameters, and ameliorated gentamicin-induced oxidative stress 17, 18. Additionally, it showed a nephroprotective effect when administered with gentamicin 19.
On the other hand, the potent antioxidant vitamin E protected against gentamicin-induced nephrotoxicity 20. However, there were no studies on the potential histopathological protection of alpha-lipoic acid and vitamin E against gentamicin-induced testicular tissue damage. Consequently, our objective was to present histological and histometric data supporting the potential protective impact of alpha-lipoic acid and vitamin E against testicular histopathology alterations induced by gentamicin administration.
MATERIALS AND METHODS
Drug preparation
Gentamicin was provided as Garamycin® ampoules brand name with a strength of 80 mg/2 ml, Schering-Plough Corporation U.S.A. Alpha lipoic acid was provided as Thioamide® ampoules generic name with a 300 mg/ 10 ml strength, E.V.A. Pharmaceutical Industries, Cairo, Egypt. Both drugs were given intraperitoneally with a 25-gauge needle with a minimal volume for each drug. Vitamin E was provided as vitamin E 1000 mg® generic name: Pharco Pharmaceuticals, Cairo, Egypt. The Vitamin E capsules were diluted in sunflower oil to achieve 1 ml per rat and delivered orally using 18-gauge soft gavage tubes. NaCl 0.9% was obtained as a sterile solution in unit doses of 0.5 ml.
Animals and study design
A minimal sample size of 4 in each group was required to detect a mean difference in the seminiferous epithelium height of 30 µm between the gentamicin-treated group and any other group with 80% power and 0.05 alpha error. A parallel experimental study was conducted, including 50 albino Wister rats weighing 200 ± 20 gm and aged 2 weeks, obtained from the animal house of the veterinary medicine faculty. The animals were acclimatized without intervention for 2 weeks at room temperature (24º C), under a 12-hour light/12-hour dark cycle, and given water and feed as recommended 21. The rats were divided into 5 groups of ten according to intervention as follows: group I received intraperitoneal gentamicin (36.5 mg/kg/day as a single dose), group II received gentamicin plus alpha-lipoic acid (100 mg/kg/day as a single dose), group III received intraperitoneal gentamicin plus intraperitoneal alpha-lipoic acid (200 mg/kg/day as a single dose), group III received intraperitoneal gentamicin plus oral vitamin E (100 mg/kg/day as a single dose), and group IV served as control and received intraperitoneal NaCl 0.9%. After oral administration, the animal was kept upright for 5 minutes to avoid drug loss. Half the animals (5 rats from each group) were euthanized on day 15 (first euthanasia day), while the remaining animals were euthanized on day 60 (second euthanasia day). All animals were euthanized under anesthesia using isoflurane inhalation 22. The study was conducted at the Departments of Pharmacology, Theriogenology, and Pathology, Faculty of Veterinary Medicine, Benha University. The faculty ethics committee approved the study with an ethical approval number of BUFVTM-080422.
Testis sampling
Immediately after euthanasia, one testis from each animal was dissected, weighed, and kept in Formation 10% (purchased from El Gomhouria Company for Trading Chemicals and Medical Appliances as formalin 34%). The formalin-preserved tissue samples were preserved in formalin for twenty-four hours and washed under running water. The washed models were dehydrated using different ascending ethanol concentrations, first using 50% concentration and finally 100% ethanol. The dehydrated tissues were fixed in xylol for 6 hours. The tissue samples were put in a soft paraffin container and left in an oven at 56°C for 12 hours. The pieces were then blocked in hard paraffin and cut into sections of about 5 microns in thickness. Paraffin was removed from the sections with absolute alcohol and washed with tap water. Sections were stained with Harris hematoxylin for 10 minutes and eosin for 5 minutes and then washed under slow-running water for 15 minutes to remove the excess stains 23. The sections were dehydrated with two changes of absolute alcohol (five minutes each), then cleared with xylol. They were superimposed with Canada balsam and protected with cover slides to be fit for microscopical examination.
Histopathological examination
Digital photos were taken using a light microscope to evaluate the histological changes among groups after various treatments at x100, x200, and x400 magnifications. The examination included entire seminiferous tubules, spermatogenic cells and spermatogenesis, seminiferous tubule epithelium, interstitial space, subcapsular area, Sertoli cells, Leydig cells, and blood vessels.
Histometric evaluation
Cross-sections were obtained for many seminiferous tubules from different sites of one testis for each rat, and the average was calculated for each histometric measure. The following measures were obtained from the cross-sections using QuPath v.0.4.2 software 24: 1 - Seminiferous tubule surface area (µm2) was taken out by tracking the tubule's circumference. 2- Seminiferous epithelium height (µm) was achieved by measuring the height of the seminiferous epithelium in five different directions of each tubule and then calculating the average. 3 - Seminiferous tubule lumen area (µm2) was achieved by obtaining the length and width of the lumen in each tubule, then calculating the lumen area as equal π multiplied by (length/2)2 and (width/2)2. 4 - Seminiferous epithelium area (µm2) was obtained as a difference between the seminiferous tubule surface area and lumen area 25-27. Pixel was converted to µm at 400X magnification with a resolution of 0.25 μm/pixel28.
Statistical analysis
Data are expressed as mean ± standard deviation (S.D.) and compared among groups using one-way ANOVA using IBM® SPSS® v.26 software. For post hoc pairwise comparisons, Tukey's test was used at a 0.05 level of significance. The mean difference (M.D.) ± standard error (S.E.) was used to show the difference between pairs in the post hoc comparison. The ANOVA assumptions were ensured, including normality, independence, and homoscedasticity 29.
RESULTS
Histopathological examination (first euthanasia day)
Typical tunica albuginea structure, intact seminiferous tubules, preserved interstitial tissue, and active spermatogenesis were observed throughout the study of gonads obtained from control group rats (Figure 1A).
Testes treated with gentamicin showed various pathological alterations as follows: some seminiferous epithelium had vacuoles in their cytoplasm (Figure 1B), a reduction of spermatogenic cells number that lined seminiferous tubules, accompanied by incomplete spermatogenesis and absence of sperm cells in the lumen of some tubules with mild inter-tubular edema (Figure 1C), prominent subcapsular blood vessels congested with subcapsular edema (Figure 1D) as well as inter-tubular edema (Figure 1E), were found. Furthermore, the seminiferous tubules had atrophy characterized by a decrease in the size of the tubules with increasing distance from one another, with variable degrees of degeneration of some seminiferous tubules (Figures F-H) in the form of a reduction of germinal epithelial cells.
In the meantime, an improvement in the testes of animals treated with gentamicin plus 100 mg/kg of alpha-lipoic acid for 2 weeks was detected. The microscopic analysis demonstrated relatively less pronounced histological alterations than the group administered with gentamicin alone over the same duration, as the majority of the seminiferous tubules were compact with one another, the tunica albuginea appeared to be expected (Figure 2A), and the spermatogenic layers appeared close to normal with typical spermatogenesis in most tubules (Figure 2B). Occasionally, the testes of only two cases showed mild degenerative changes in the spermatogenic cells of some seminiferous tubules in the form of swollen, pale, and vacuolated cytoplasm of the lining epithelium (Figure 2C).
The histopathological examination of the testes treated with gentamicin plus 200 mg/kg of the alpha-lipoic acid group revealed marked improvement with complete spermatogenic cells. Most of the seminiferous tubules restored their typical structure (Figure 2D), and the spermatogenesis processes are standard; only mild inter-tubular edema (Figure 2E), as well as degeneration of spermatogenic series, was demonstrated in some seminiferous tubules of the testes of one examined animal (Figure 2F).
On the other hand, microscopically examining the testes of animals treated with gentamicin plus vitamin E revealed less prominent changes than those treated with gentamicin alone. The majority of the seminiferous tubules were compact with one another, and the tunica albuginea approximated the typical structure in most examined animals (Figure 2G); hence, except for specific seminiferous tubules that had modest degeneration of the lining epithelial cells along with sub-capsular and inter-tubular edema, most of the seminiferous tubules recovered their standard histological architecture. The spermatogonia exhibited signs of degradation, characterized by the presence of cytoplasmic vacuoles and the shedding of many spermatocytes into the lumen of the seminiferous tubules, along with some seminiferous tubular epithelial necrosis with incomplete spermatogenesis (Figure 2H).
Histopathological examination (second euthanasia day)
The histopathological examination of testicular tissues in all investigated groups showed the standard histological structure of tunica albuginea, seminiferous tubules, and interstitial tissue (Figure 3A) with typical spermatogenesis (Figure 3B). However, mild degenerative changes in seminiferous tubules in association with mild sub-capsular and inter-tubular edema (Figure 3C & 3D) were demonstrated in some seminiferous tubules of rats treated with gentamicin alone
Histometric results
As shown in Table 1, the histometric evaluation of testes obtained from rats on the first euthanasia day revealed that seminiferous tubule diameter did not significantly differ among groups treated with gentamicin, gentamicin + 100 mg/kg of alpha-lipoic acid, and the control group. However, groups treated with gentamicin + 200 mg/kg of alpha-lipoic acid or gentamicin + vitamin E exhibited larger diameters with M.D. ± S.E. of 78.9 ± 11.4 104.6 ± 11.4, respectively, compared to the control.
Seminiferous tubule height was reduced in rats treated with gentamicin compared to the control (16.6 ± 4.1). However, the group treated with 100 mg/kg of alpha-lipoic acid showed a nonsignificant mean difference compared to the control. Moreover, groups treated with gentamicin + 200 mg/kg of alpha-lipoic acid or gentamicin + vitamin E had higher values than the control (21.5 ± 4.1, 23.9 ± 4.1, respectively).
Seminiferous tubule cross-sectional area did not significantly differ among groups treated with gentamicin, gentamicin + 100 mg of alpha-lipoic acid, and the control group. However, groups treated with gentamicin + 200 mg/kg of alpha-lipoic acid or gentamicin + vitamin E exhibited larger areas (47293.4 ± 2598, 53360.6 ± 2598, respectively) than the control group.
The luminal area was increased (10196.6 ± 2777.9), and the seminiferous epithelial area was reduced (4855.8 ± 2150.7) with gentamicin treatment compared to the control group. These changes were normalized with 100 mg/kg of alpha-lipoic acid co-treatment. However, higher values were obtained with vitamin E or 200 mg/kg of alpha-lipoic acid treatment.
The seminiferous epithelial area ratio and seminiferous epithelial height ratio were reduced with gentamicin treatment compared to the control group (0.134 ± 0.03, 0.065 ± 0.015, respectively). However, the proportions were normalized with 100 mg or 200 mg/kg of alpha-lipoic acid or vitamin E co-treatment, and there was no significant difference between the co-treated groups and the control group.
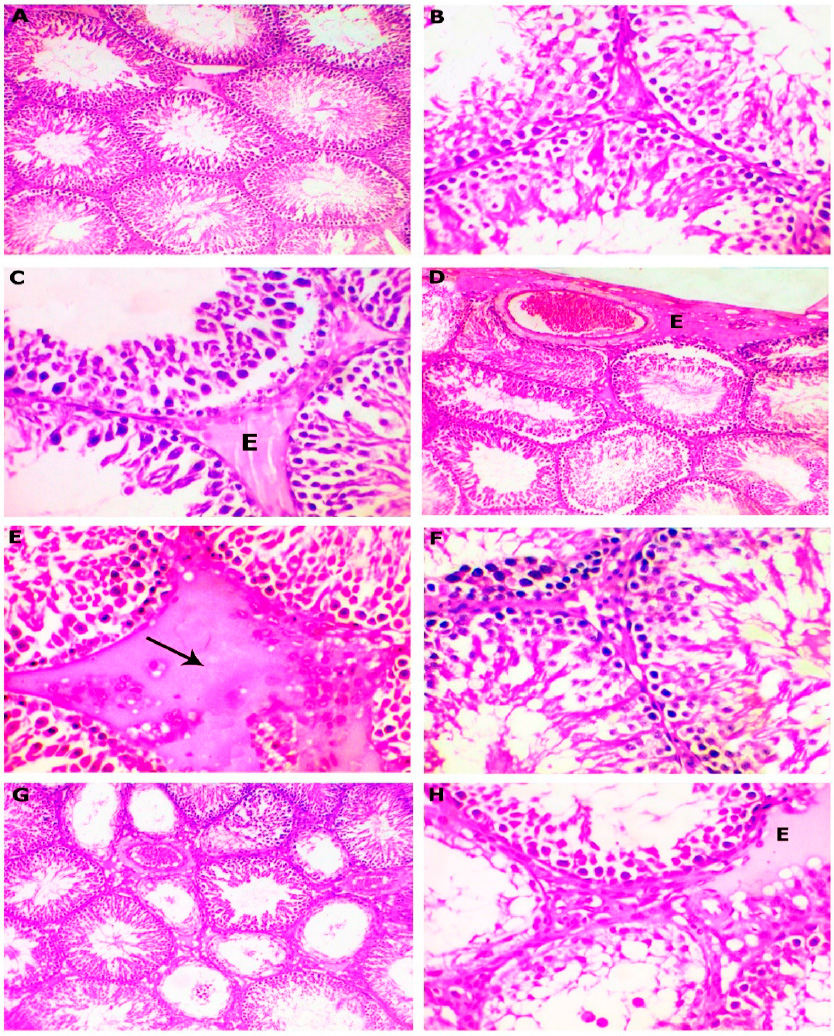
Figure 1: H&E stained section of testes obtained from rat, post administration of placebo (A) and gentamicin for 2 weeks (B-H), showing (A), typical histological structure of seminiferous tubules with active spermatogenesis (x100), (B) swollen, pale, and vacuolated cytoplasm of the lining epithelium of some seminiferous tubules (x400), (C) reduction in the number of spermatogonial cells lined seminiferous tubules with incomplete spermatogenesis and absence of spermatozoa in the lumen of some tubules with mild inter-tubular edema (E, x400), (D) marked congestion of subcapsular blood vessels with subcapsular edema (x100), (E) inter-tubular edema (arrow, x400), (F) degenerated germ cells (x400), (G), degeneration and necrosis of the lining epithelial cells of some seminiferous tubule (x100), (H), extensive degeneration of the lining epithelium of seminiferous tubules with mild inter-tubular edema (E, x400).
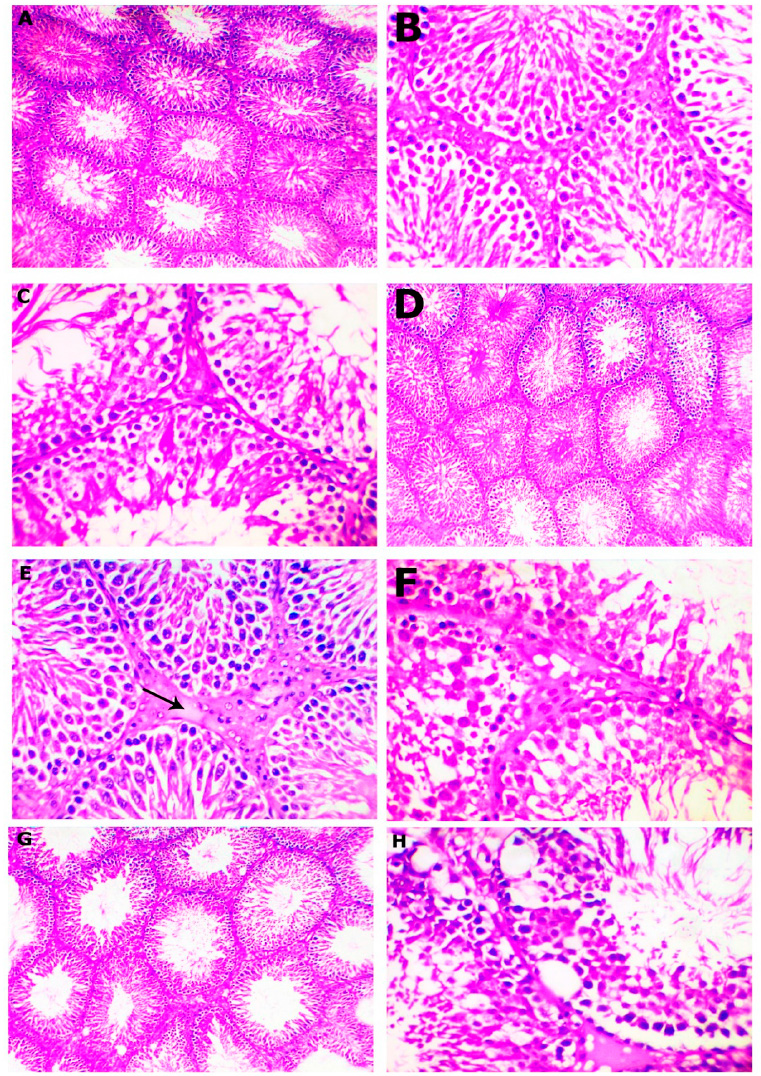
Figure 2: H&E-stained section of testes obtained from rat, post administration of gentamicin plus 100 mg A.L.A. (A-C), and gentamicin plus 200 mg A.L.A. (D-F) and gentamicin plus vitamin E (G-H) for 2 weeks, showing (A), normal seminiferous tubules and compact with each other (x100), (B), most of the seminiferous tubules showed regular spermatogenic layers and normal spermatogenesis (x400), (C), swollen, pale, and vacuolated cytoplasm of the lining epithelium of some seminiferous tubules (x400), (D), Most of the seminiferous tubules restored their typical histological structure (x100), (E), normal spermatogenesis with mild inter-tubular edema (arrow, x400), (G), the seminiferous tubules were compact with each other. The spermatogenic layers appeared somewhat normal (x100), (H), and cytoplasmic vacuolization with necrosis of some seminiferous tubular epithelium (x400).
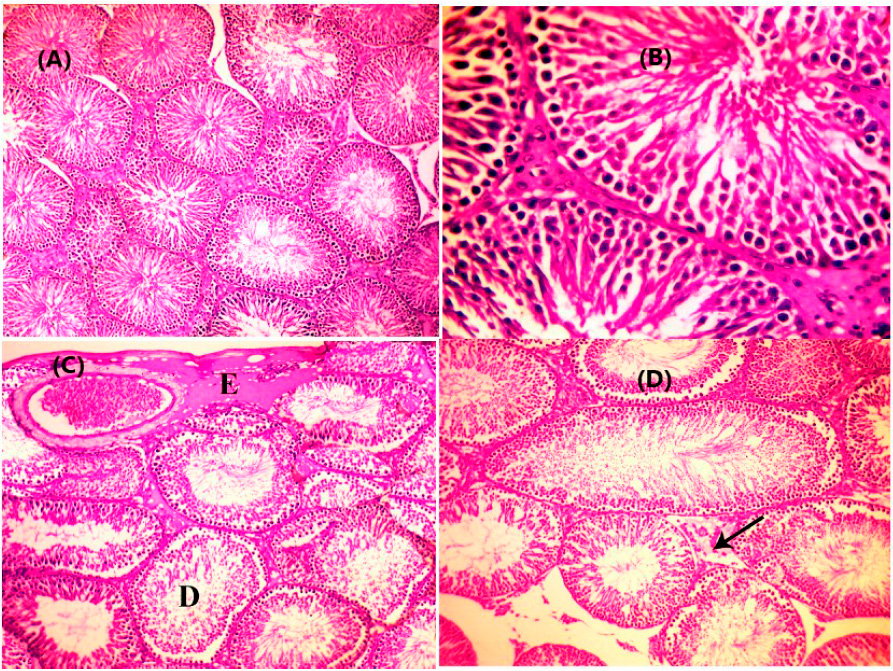
Figure 3: Testes of rats treated with gentamicin were obtained on the second euthanasia day; (A): Testes showed typical histological structures of seminiferous tubules and interstitial tissues. H&E stain x 100. (B): Testes of rats showing normal spermatogenesis. H&E stain x 400. (C): Testes of rats show sub-capsular edema "E" with degeneration of spermatogenic cells of some seminiferous tubules "D." H&E stain x 100. (D): Testes of rats show inter-tubular edema (arrow) with degeneration of spermatogenic cells of seminiferous tubules "D." H&E stain x 100.
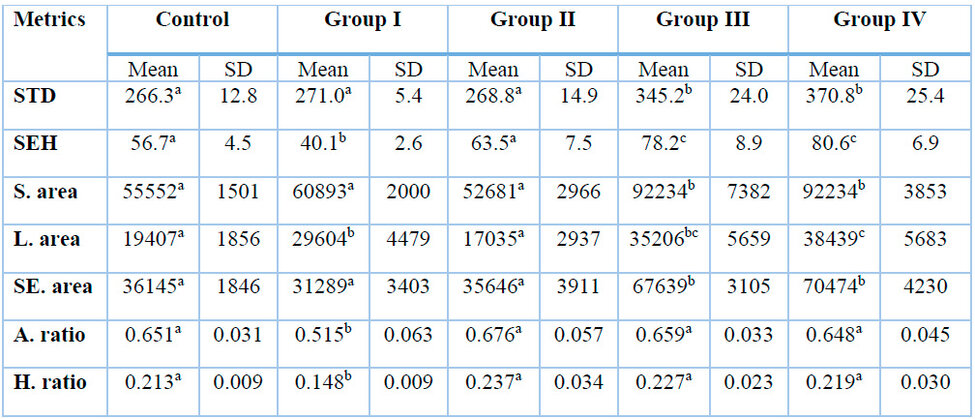
Table 1: Histometric evaluation of rat testes treated with gentamicin with/without alpha-lipoic acid.
Group I was treated with gentamicin alone, group II was treated with gentamicin plus 100 mg/kg of alpha-lipoic acid; group III was treated with gentamicin plus 100 mg/kg of alpha-lipoic acid, group IV was treated with gentamicin plus vitamin E, A. ratio; area ratio equals lumen area divided by seminiferous tubule area, H. ratio; height ratio equals seminiferous epithelium height divided by seminiferous tubule diameter, L. area; lumen cross-sectional area (µ), S. area; seminiferous tubule area (µ2), S.E. Area: seminiferous epithelium area (µ2), S.E.H., seminiferous epithelium height (µ), S.T.D.; seminiferous tubule diameter (µ). A one-way ANOVA test was carried out, followed by post hoc Tukey's test for pairwise comparison; values in the same raw with different superscript letters indicate significant differences at 0.05 level.
DISCUSSION
This study is the initial investigation aimed at examining the histopathological evidence about the protective impact of alpha-lipoic acid against gonadotoxicity induced by gentamicin. In several studies, it has been observed that administering gentamicin leads to a reduction in testis weight, alteration of sperm parameters, a decline in serum testosterone levels, and an elevation in testicular oxidative stress. Some studies evaluated the testicular histopathological effect of gentamicin and found variable detrimental effects such as reduced germ cells, spermatogenic cell necrosis, seminiferous tubule degeneration with atrophy, incomplete spermatogenesis, interstitial space expansion, veins congestion, and decreased seminiferous epithelial thickness 2, 7, 9, which is consistent with the present study findings. Some antioxidants, such as lycopene, were histopathologically investigated for their potential protective effect against gentamicin-related testicular damage and showed favorable outcomes. 7. Interestingly, each alpha-lipoic acid and vitamin E showed a promising protective effect against testicular adverse effects of gentamicin through morphological and biochemical evidence in recent studies 17, 18. So, the histopathological effects of gentamicin alone compared to either the gentamicin-alpha-lipoic acid combination or the gentamicin-vitamin E combination were evaluated. A comparable protective effect of alpha-lipoic acid and vitamin E and a dose-dependent protection of alpha-lipoic acid on the histological structure of rat testes treated with gentamicin were observed compared to the control. However, with 100 mg/kg of alpha-lipoic acid, the spermatogenic layers appeared somewhat normal with typical spermatogenesis in most tubules, which might be a sufficient dose to exhibit reliable protection. Consistently, only one recent study evaluated the protective effect of alpha-lipoic acid against the testicular harmful impacts of gentamicin, seeking morphological, hormonal, and histopathological evidence. The study reported good protection of alpha-lipoic acid on the histological structure of the testis. However, the study used alpha-lipoic acid with 600 mg/kg of oral suspension dose 17. Our results showed that gentamicin harmed spermatogenesis, which could be mitigated with either alpha-lipoic acid or vitamin E treatment. In agreement with us, a study reported decreased sperm count, altered spermatogenesis, and spermatogonia necrosis among rats treated with 5 mg/kg of gentamicin 30. In another study, alpha-lipoic acid could restore sperm count and abnormalities associated with gentamicin treatment 18. The histometric analysis of the present research showed an increase in the seminiferous tubule diameters among rats co-treated with high-dose alpha-lipoic acid or vitamin E, accompanied by a rise in other metrics. However, the area and height ratios showed nonsignificant differences among all groups except those treated with gentamicin alone, which exhibited lower ratios. Consistently, the negative effect of gentamicin on seminiferous tubule epithelial height was demonstrated in the histopathological examination of the present study. In agreement with our findings, a very recent study evaluated the effect of gentamicin on the seminiferous tubule epithelial thickness and reported a significant reduction with gentamicin treatment 31. Finally, it was noticed that gentamicin-induced testicular adverse effects were reversible as the testicular tissues are highly regenerative 32. Consistently, the reversibility of the gentamicin effect on testes was demonstrated in testis weight and hormone levels 18, 33.
Limitations
The authors faced a few limitations: using NaCl 0.9% as a control treatment rather than the original solvent of drugs used due to the unavailability of these solvents in pure forms. Another limitation was the relatively small sample size used in this experiment. However, it provided sufficient power, which was 80%.
CONCLUSIONS
In summary, it can be concluded that both alpha-lipoic acid and vitamin E have a protective role in maintaining the testicular architecture, preserving the integrity of seminiferous tubule epithelial cells, and sustaining the process of spermatogenesis in the presence of gentamicin administration. The effects of both medications have a comparable nature, rendering them suitable for application in human investigations, mainly due to their established safety profiles.
Author Contributions: Conceptualization, A.E. and M.K.; methodology, H.E., A.A., and H.M.; software, A.A. and A.E.; validation, A.E. and M.K.; formal analysis, H.E. and A.A.; investigation, A.A.; resources, A.E. and A.A.; data curation, H.E. and A.A.; writing—original draft preparation, H.E. and A.A.; writing—review and editing, H.E., A.E., and A.F.; visualization, A.A.; supervision, A.E., M.K., A.F.; project administration, A.E.
Funding: This research received no external funding.
Institutional Review Board Statement: The study was approved by the ethics committee of the faculty of veterinary medicine with an ethical approval number of BUFVTM-080422.
Data Availability Statement: The data supporting this study's findings are available from the corresponding author upon request.
Acknowledgments: I would like to acknowledge the assistance of the academic staff of the pathology department in the histopathological examinations. Their contributions greatly enriched the depth and rigor of this study.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: A review of literature. Journal of human reproductive sciences. 2015;8(4):191.
2. Aly HA, Hassan MH. Potential testicular toxicity of gentamicin in adult rats. Biochemical and biophysical research communications. 2018;497(1):362-7.
3. Darlow CA, da Costa RM, Ellis S, Franceschi F, Sharland M, Piddock L, et al. Potential antibiotics for the treatment of neonatal sepsis caused by multidrug-resistant bacteria. Pediatric Drugs. 2021;23:465-84.
4. Goodlet KJ, Benhalima FZ, Nailor MD. A systematic review of single-dose aminoglycoside therapy for urinary tract infection: is it time to resurrect an old strategy? Antimicrobial agents and chemotherapy. 2019;63(1):e02165-18.
5. Siddiqui FG, Jung S, Ali A, Prakash A, Rais H, Kumar A. Ampicillin & Gentamicin V/S 3rd Generation Cephalosporin for the Management of Community-Acquired Pneumonia in Children; A Comparative Analysis. Journal of Pharmaceutical Research International. 2021:29-35.
6. Elsawah HK, Kandiel MM, Amin AA, Mokhimar HM, El Mahmoudy A. Gentamicin and amikacin adversely affect male infertility indicated by pharmacological, andrological and pathological evidence. International Journal of Basic & Clinical Pharmacology. 2020;9(2):218.
7. Aly H. Testicular toxicity of gentamicin in adult rats: Ameliorative effect of lycopene. Human & experimental toxicology. 2019;38(11):1302-13.
8. Kim SH, Lee IC, Baek HS, Shin IS, Moon C, Kim SH, et al. Melatonin prevents gentamicin‐induced testicular toxicity and oxidative stress in rats. Andrologia. 2014;46(9):1032-40.
9. Khaki A, Ghaffari Novin M, Khaki A, Fathiazad F, Khabiri M, Hossinchi J. Ultra structural study of gentamicin and ofloxacin effect on testis tissue in rats: Light and transmission electron microscopy. Afr J Pharm Pharmacol. 2009;3(4):105-9.
10. Karaman M, Budak H, Çiftci M. Amoxicillin and gentamicin antibiotics treatment adversely influence the fertility and morphology through decreasing the Dazl gene expression level and increasing the oxidative stress. Archives of physiology and biochemistry. 2019;125(5):447-55.
11. Morales AI, Detaille D, Prieto M, Puente A, Briones E, Arévalo M, et al. Metformin prevents experimental gentamicin-induced nephropathy by a mitochondria-dependent pathway. Kidney international. 2010;77(10):861-9.
12. Lesniak W, Pecoraro VL, Schacht J. Ternary complexes of gentamicin with iron and lipid catalyze formation of reactive oxygen species. Chemical research in toxicology. 2005;18(2):357-64.
13. Dutta S, Sengupta P, Slama P, Roychoudhury S. Oxidative stress, testicular inflammatory pathways, and male reproduction. International Journal of Molecular Sciences. 2021;22(18):10043.
14. Tran LV, Malla BA, Kumar S, Tyagi AK. Polyunsaturated fatty acids in male ruminant reproduction-a review. Asian-Australasian journal of animal sciences. 2017;30(5):622-37.
15. Khosrowbeygi A, Zarghami N. Fatty acid composition of human spermatozoa and seminal plasma levels of oxidative stress biomarkers in subfertile males. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2007;77(2):117-21.
16. Prahalathan C, Selvakumar E, Varalakshmi P. Protective effect of lipoic acid on adriamycin-induced testicular toxicity. Clinica chimica acta. 2005;360(1-2):160-6.
17. Yahya K, Hassan AH, Nadhem H. Evaluation the Effect of Gentamicin on Fertility of Male Rats & Probable Protective Role of Lipoic Acid. Indian Journal of Public Health Research & Development. 2019;10(6).
18. Elsawah H, Mokhimar HM, Kandiel MM, Amin A, Farid AS, El-Mahmoudy A. The ameliorative effect of α-lipoic acid on testicular dysfunction induced by gentamicin. Benha Veterinary Medical Journal. 2022;42(2):104-8.
19. Tahira A, Saleem U, Mahmood S, Hashmi FK, Hussain K, Bukhari NI, et al. Evaluation of protective and curative role of α-lipoic acid and selenium in gentamicin-induced nephrotoxicity in rabbits. Pakistan Journal of Pharmaceutical Sciences. 2012;25(1).
20. Derakhshanfar A, Bidadkosh A, Kazeminia S. Vitamin E protection against gentamicin-induced nephrotoxicity in rats: a biochemical and histopathologic study. 2007.
21. Olfert ED, Cross BM, McWilliam AA. Guide to the care and use of experimental animals: Citeseer; 1993.
22. Nakatsu N, Igarashi Y, Aoshi T, Hamaguchi I, Saito M, Mizukami T, et al. Isoflurane is a suitable alternative to ether for anesthetizing rats prior to euthanasia for gene expression analysis. The Journal of Toxicological Sciences. 2017;42(4):491-7.
23. Drury R, Wallington E. Carleton's Histological Techniques (6th edn.). Carleton's Histological Techniques (6th edn) New York, Toronto, U.S.A.: Oxford University Press; 1980. p. 126 - 7.
24. Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, et al. QuPath: Open source software for digital pathology image analysis. Scientific reports. 2017;7(1):1-7.
25. Golalipour M, Azarhoush R, Ghafari S, Gharravi A, Fazeli S, Davarian A. Formaldehyde exposure induces histopathological and morphometric changes in the rat testis. Folia morphologica. 2007;66(3):167-71.
26. Montoto LG, Arregui L, Sánchez NM, Gomendio M, Roldan ER. Postnatal testicular development in mouse species with different levels of sperm competition. Reproduction. 2012;143(3):333.
27. Kumar A, Nagar M. Histomorphometric study of testis in deltamethrin treated albino rats. Toxicology reports. 2014;1:401-10.
28. Hein AL, Mukherjee M, Talmon GA, Natarajan SK, Nordgren TM, Lyden E, et al. QuPath digital immunohistochemical analysis of placental tissue. Journal of Pathology Informatics. 2021;12(1):40.
29. Emerson RW. ANOVA assumptions. SAGE Publications Sage CA: Los Angeles, CA; 2022. p. 585-6.
30. Fetouh FA, Azab AES. Ameliorating effects of curcumin and propolis against the reproductive toxicity of gentamicin in adult male guinea pigs: Quantitative analysis and morphological study. American Journal of Life Sciences. 2014;2(3):138-49.
31. Mardatillah M, Wurlina W, Yudaniayanti IS, Plumeriastuti H, Primarizky H, Hamid IS. Moringa oleifera leaf extract restored the diameter and epithelium thickness of the seminiferous tubules of rat (Rattus norvegicus) injected with gentamicin. Ovozoa: Journal of Animal Reproduction. 2022;11(1):15-21.
32. Koizumi T, Li ZG. Role of oxidative stress in single‐dose, cadmium‐induced testicular cancer. Journal of Toxicology and Environmental Health, Part A Current Issues. 1992;37(1):25-36.
33. Carageorgiou HK, Stratakis CA, Damoulis PD, Varonos DD, Messari ID, Sideris AC, et al. Reversible plasma testosterone levels reduction after gentamicin administration and freund's adjuvant arthritis in rats. Indian journal of physiology and pharmacology. 2005;49(4):443.
Received: October 9th 2023/ Accepted: January 15th 2024 / Published:15 February 2024
Citation: Elsawah H., Amin A., Mokhimar H., Kandiel M., Farid A., El-Mahmoudy A. Evaluating the protective effect of alpha-lipoic acid against gentamicin-induced gonado toxicity indicated by histopathology. Revis Bionatura 2024; 1 (1) 73. http://dx.doi.org/10.21931/BJ/2024.01.01.73
Additional information Correspondence should be addressed to [email protected]
Peer review information. Bionatura thanks anonymous reviewer(s) for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
All articles published by Bionatura Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Publisher's Note: Bionatura stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2024 by the authors. They were submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
